
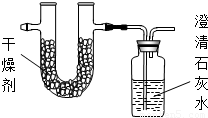
| ʵ��ǰ | ʵ��� | |
| �������+U�ܣ������� | 101.1g | 103.62g |
| ��ʯ��ˮ+���ƿ�������� | 312.0g | 317.28g |
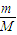 =
= ��֪����ܶ�֮�ȵ�����Է�������֮�ȣ�ȷ����������Է�������Ϊ2×43=86��
��֪����ܶ�֮�ȵ�����Է�������֮�ȣ�ȷ����������Է�������Ϊ2×43=86�� =0.02mol��
=0.02mol�� =0.14mol��n��H��=0.28mol��
=0.14mol��n��H��=0.28mol��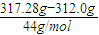 =0.12mol��
=0.12mol�� =14molHԭ�ӣ�����
=14molHԭ�ӣ����� =6molCԭ�ӣ�
=6molCԭ�ӣ�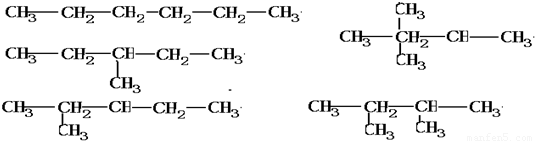 ��
��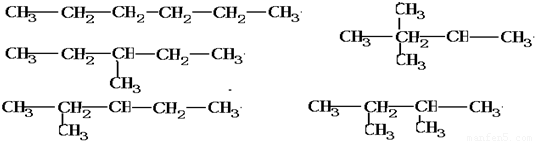 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
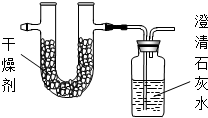
 ��һ���������ʣ�Ϊ�˲ⶨ����ɣ��������²��ԣ�
��һ���������ʣ�Ϊ�˲ⶨ����ɣ��������²��ԣ�| ʵ��ǰ | ʵ��� | |
| �������+U�ܣ������� | 101.1g | 103.62g |
| ��ʯ��ˮ+���ƿ�������� | 312.0g | 317.28g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ʵ��ǰ | ʵ��� | |
| �������+U�ܣ������� | 101.1g | 103.62g |
| ��ʯ��ˮ+���ƿ�������� | 312.0g | 317.28g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com