��2011?����ģ�⣩ú��������úΪԭ�ϣ�������ѧ�ӹ�ʹúת��Ϊ���塢����ȼ���Լ����ֻ�����Ʒ�Ĺ�ҵ���̣�
��1����ˮ����ͨ�����ȵ�̿���ɲ���ˮú������ӦΪ��C��s��+H
2O��g��?CO��g��+H
2��g����H=+131.3kJ/mol
�ٸ÷�Ӧ�ܷ��Է�������
�¶�
�¶�
�йأ�
����һ���¶��£���һ���ݻ�������ܱ������з���������Ӧ���������жϸ÷�Ӧ�Ѵﻯѧƽ��״̬����
ABD
ABD
��
A�������е�ѹǿ���� B��������������ܶȲ���
C��c��CO��=c��H
2�� D��1 molH-H�����ѵ�ͬʱ����2mol H-O��
���ں����ܱ������м���1mol C��s����1mol H
2O��g����һ���¶��³�ַ�Ӧ�ﵽƽ�⣬��������akJ����a=
��
��
131.3�����������������=����������ʼʱ�������2mol C��s����1mol H
2O��g������ַ�Ӧ�ﵽƽ�⣬��������Ϊb kJ����b
=
=
a�����������������=������
��2����һ����CO��g����H
2O��g���ֱ�ͨ�뵽���Ϊ2L�ĺ����ܱ������н��з�Ӧ��CO��g��+H
2O��g��?CO
2��g��+H
2��g��
|
ʵ���� |
�¶�/�� |
��ʼ��/mol |
ƽ����/mol |
�ﵽƽ������ʱ��/min |
|
CO |
H2O |
H2 |
CO2 |
|
1 |
650 |
4 |
2 |
1.6 |
1.6 |
5 |
|
2 |
830 |
1 |
4 |
0.8 |
0.8 |
3 |
|
3 |
830 |
a |
b |
c |
d |
t |
��ʵ��1�е�v��H
2����ʾ�ķ�Ӧ����Ϊ
0.16mol/��L?min��
0.16mol/��L?min��
��
��830��ʱ���������г���2mol CO��10mol H
2O��g������Ӧ�ﵽƽ���CO��ת����Ϊ
83.33%
83.33%
��
����ʵ��3Ҫ�ﵽ��ʵ��2���Ƶ�ƽ��״̬���������ʵ����������ֱ���ȣ�����t��3min����a��bӦ����Ĺ�ϵ��
b=4a��a��1����ʹ�ô�����
b=4a��a��1����ʹ�ô�����
���ú�a��b����ѧʽ��ʾ��

 E (g) ������Ӧ���е�4minʱ�ﵽƽ�⣬��ʱ��֪M��Ũ��Ϊ0.2 mol��L��1������˵������ȷ����
E (g) ������Ӧ���е�4minʱ�ﵽƽ�⣬��ʱ��֪M��Ũ��Ϊ0.2 mol��L��1������˵������ȷ����  �����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӽݾ��㽭��ѧ������ϵ�д�
 ��2013?������ģ�⣩��һ���¶��£���2L����̶����ܱ������м���1molHI��2HI?H2��g��+I2��g����H��0��H2�����ʵ�����ʱ��ı仯��ͼʾ������˵����ȷ���ǣ�������
��2013?������ģ�⣩��һ���¶��£���2L����̶����ܱ������м���1molHI��2HI?H2��g��+I2��g����H��0��H2�����ʵ�����ʱ��ı仯��ͼʾ������˵����ȷ���ǣ������� H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯�����
H2��g��+CO2��g����ƽ�ⳣ�����¶ȵı仯����� 2CO��g��ƽ�ⳣ��K��
2CO��g��ƽ�ⳣ��K�� CO��g��+H2��g�� ƽ�ⳣ��K1��
CO��g��+H2��g�� ƽ�ⳣ��K1�� H2��g��+CO2��g�� ƽ�ⳣ��K2��
H2��g��+CO2��g�� ƽ�ⳣ��K2��
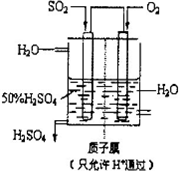 ��2010?�ij�ģ�⣩���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裮
��2010?�ij�ģ�⣩���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裮