
���� ��1����п����Ҫ�ɷ�ΪZnS���ڿ����и��¼�����п������ZnO��SO2�����ݹ������������������㼴�ɣ�
��2��SO2���������Ʒ�Ӧ������Na2SO3����ͨ����ѧ����ʽ�ļ���ó���������SO2���������Ʒ�Ӧ������NaHSO3����ͨ����ѧ����ʽ�ļ���ó����������Ƚϼ���Ӧ��ù���������ʵ������֮��Ĺ�ϵ���Խ������ۣ��ڸ���SԪ���غ��������ϵ������Ե����Ķ��ټ��ɣ�����Ũ�ȣ�
��� �⣺��1����ZnS��������x����
2ZnS+3O2=2ZnO+2SO2 ���ٵĹ�������
194 161 32
x 1.56-1.32
��$\frac{194}{x}=\frac{32}{1.56-1.32}$ ���x=1.455g������ZnS����������=$\frac{1.445}{1.56}$=92.63%��
����Ʒ�к���п����������92.63%��
��2��n��SO2��=$\frac{4.48}{22.4}$=0.2mol
�ټ��趼����Na2SO3 SO2+2NaOH=Na2SO3+H2O
1 126
0.2 25.2
�ʼ��趼����Na2SO3 ������Ϊ25.2g
�ڼ��趼����NaHSO3 SO2+NaOH�TNaHSO3
1 104
0.2 20.8
�ʼ��趼����NaHSO3������Ϊ20.8g
A������ˮ����23gʱ���� 20.8g��25.2g֮�䣬����ˮ����ΪNa2SO3�� NaHSO3
��Na2SO3�� NaHSO3�ֱ�Ϊxmol��ymol��������ԭ���غ��n��SO2��=n��Na2SO3��+n��NaHSO3��=x+y=0.2��
126x+104y=23��
�ɢ٢ڽ��x=0.1��y=0.1��������Ԫ���غ㣬��n��NaOH��=2n��Na2SO3��+n��NaHSO3��=2x+y=0.3mol����c��NaOH��=$\frac{0.3mol}{0.2}$=0.15mol/L��
B..����ˮ����26gʱ������ 20.8g��25.2g֮�䣬��NaOH����ֻ�����˷�ӦSO2+2NaOH=Na2SO3+H2O
1 80 126
0.2 16 25.2
mʣ��NaOH��=26-25.6=1.4g��m��NaOH��=16+1.4=17.4g��c��NaOH��=$\frac{\frac{17.4}{40}}{0.2}$=2.175mol/L
����ԭNaOH��ҺA��B�����ʵ���Ũ�ȷֱ�Ϊ0.15mol/L��2.175mol/L��
���� ������һ������Ԫ���Լ�������ļ����⣬���ֽ���˼�룺Ԫ���غ㡢����Ӧ�����Ӧ���ǽ���Ĺؼ����Ѷȴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ų�Y kJ | B�� | �ų���5X-0.5Y�� kJ | C�� | �ų���10X-Y�� kJ | D�� | ���գ�10X-Y�� kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
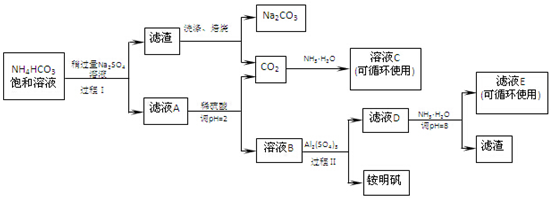
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
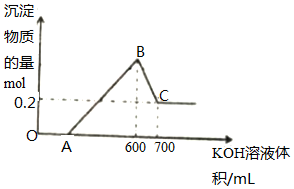 ��һ���������ۡ�������������þ�Ļ������뵽300mL4mol/L��ϡ�����У����ɱ�״����6.72L���壮��Ӧ�����Һ�еμ�һ�����ʵ���Ũ�ȵ�KOH��Һʱ�����ó��������ʵ�����mol����μ�KOH��Һ�������mL��֮��Ĺ�ϵ��ͼ��ʾ��
��һ���������ۡ�������������þ�Ļ������뵽300mL4mol/L��ϡ�����У����ɱ�״����6.72L���壮��Ӧ�����Һ�еμ�һ�����ʵ���Ũ�ȵ�KOH��Һʱ�����ó��������ʵ�����mol����μ�KOH��Һ�������mL��֮��Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a% | B�� | $\frac{3}{4}$a% | C�� | $\frac{6��1-a%��}{7}$ | D�� | $\frac{12��1-a%��}{13}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ٳ�������ʵ�����CO2��H2�ﵽ�µ�ƽ��״̬��CO������������� | |
| B�� | �����������ٳ���1molCO��ƽ�������ƶ���H2������������� | |
| C�� | ����ʼʱ����1.2mol H2O��g����0.6mol CO���ﵽƽ��ʱH2������������� | |
| D�� | ����ʼʱ����0.9mol H2O��g����0.6mol CO��0.7mol CO2��0.8molH2�����ʱ����=���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 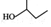 ������Ϊ2-��-1-���� ������Ϊ2-��-1-���� | B�� | ����ϩ�;���ϩ���ܷ����ӳɷ�Ӧ | ||
| C�� | C3H2Cl6 ��4��ͬ���칹�� | D�� | C2H4��C4H8һ����ͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | Ŀ�� | ���뷽�� | ԭ�� |
| A | ��ȡ��ˮ�еĵ� | �Ҵ���ȡ | �����Ҵ��е��ܽ�ȱ�ˮ�д� |
| B | ��ȥ�����е�̼����� | ���� | ��������ȶ��Դ���̼����� |
| C | ����KNO3��NaCl | �ؽᾧ | ����ص��ܽ�ȴ����Ȼ��� |
| D | ���������������Ҵ� | ��Һ | ���������ܶȴ����Ҵ� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ�Ľṹ��ʽΪCH2CH2 | |
| B�� | ����������1mol CH4��3mol Cl2��Ӧ���Ƶ�1mol CHCl3 | |
| C�� | 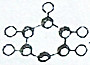 Ϊ�����ӵı���ģ�� Ϊ�����ӵı���ģ�� | |
| D�� | HC��C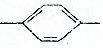 CH�TCHCH3������̼ԭ���п��ܶ���ͬһ��ƽ���� CH�TCHCH3������̼ԭ���п��ܶ���ͬһ��ƽ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com