
��14�֣�
0.80g CuSO4��5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��

��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ______________��Ҫ��д���ƶϹ��̣���
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ______________���Ѹú�ɫ��ĩ�ܽ���ϡ�����У���Ũ������ȴ���о����������þ���Ļ�ѧʽΪ_________������ڵ�����¶���_____________��
��3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ________________��
��4����0.10mol��L-1����ͭ��Һ�м�����������ϡ��Һ��ֽ��裬��dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱ��c��Cu2+��=________________mol��L-1��Ksp[Cu��OH��2]=2.2��10-20����
����0.1mol��L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�H+Ũ����_______________mol��L-1��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011����ͨ�ߵ�ѧУ����ȫ��ͳһ���Ի�ѧ�����¿α꣩ ���ͣ�ѡ����
0.80g ��5
��5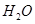 ��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��
��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��

��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ______________��Ҫ��д���ƶϹ��̣���
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ______________���Ѹú�ɫ��ĩ�ܽ���ϡ�����У���Ũ������ȴ���о����������þ���Ļ�ѧʽΪ_________������ڵ�����¶���_____________��
��3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ________________��
��4����0.10mol��L-1����ͭ��Һ�м�����������ϡ��Һ��ֽ�����dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱ��c��Cu2+��=________________mol��L-1��Kap[Cu��OH��2]=2.2��10-20����
����0.1mol��L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�H+Ũ����_______________mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿����� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
0.80g CuSO4��5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��

��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ______________��
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ______________���Ѹú�ɫ��ĩ�ܽ���ϡ�����У���Ũ������ȴ���о����������þ���Ļ�ѧʽΪ_________������ڵ�����¶���_____________��
��3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ________________��
��4������0.1mol��L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�H+Ũ����_______________mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�
0.80g CuSO4��5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ�����ͼ��ʾ��
��ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ______________��Ҫ��д���ƶϹ��̣���
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ______________���Ѹú�ɫ��ĩ�ܽ���ϡ�����У���Ũ������ȴ���о����������þ���Ļ�ѧʽΪ_________������ڵ�����¶���_____________��
��3������������������ˮ��Ӧ����һ�ֻ�����û������Ũ��Һ��Cu�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽΪ________________��
��4����0.10mol��L-1����ͭ��Һ�м�����������ϡ��Һ��ֽ��裬��dz��ɫ������ͭ�������ɣ�����Һ��pH=8ʱ��c��Cu2+��=________________mol��L-1��Ksp[Cu��OH��2]=2.2��10-20����
����0.1mol��L-1����ͭ��Һ��ͨ�����H2S���壬ʹCu2+��ȫ����ΪCuS����ʱ��Һ�е�H+Ũ����_______________mol��L-1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com