
����Ŀ����1������Һ��������Ҫ�ɷ�֮һ�Ƕ���(C4H10)����1kg������ȫȼ�����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ5��104kJ����д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ___��
��2����ѧ������̫���ֽܷ�ˮ���ɵ������ڴ����������������̼��Ӧ���ɼ״�����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�ء���֪H2(g)��CO(g)��CH3OH(l)��ȼ���Ȧ�H�ֱ�Ϊ��285.8kJ/mol����283.0kJ/mol�ͣ�726.5kJ/mol����ش��������⣺
����̫���ֽܷ�10molˮ���ĵ�������___kJ��
�ڼ״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ_____��
���о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���壬��֪��
2SO2(g)��O2(g)=2SO3(g) ��H����196.6 kJ/mol
2NO(g)��O2(g)=2NO2(g) ��H����113.0kJ/mol
��ӦNO2(g)��SO2(g)=SO3(g)��NO(g)�Ħ�H��___kJ/mol��
���𰸡�C4H10(g)+13/2O2(g)=4CO2(g)+5H2O(l) ��H=-2900kJ/mol 2858 CH3OH(l)��O2(g)=CO(g)��2H2O(l) ��H����443.5kJ/mol -41.8
��������
(1)���������������1mol������ȫȼ�շ���д���Ȼ�ѧ����ʽ��
(2)��������ȼ���ȿ�֪ˮ�ֽ����յ�������Ȼ�����û�ѧ�������뷴Ӧ�ȵĹ�ϵ�����㣻
�ڸ���CO��CH3OH��ȼ��������д�ȷ���ʽ�������ø�˹�����������״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��
����֪����2SO2(g)+O2(g)![]() 2SO3(g)��H1= -196.6kjmol-1 ��2NO(g)+O2(g)
2SO3(g)��H1= -196.6kjmol-1 ��2NO(g)+O2(g)![]() 2NO2(g)��H2= -113.0kJmol-1�����ø�˹���ɽ�
2NO2(g)��H2= -113.0kJmol-1�����ø�˹���ɽ�![]() �ɵ÷�Ӧ�ȡ�
�ɵ÷�Ӧ�ȡ�
(1)��1kg������ȫȼ�����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ5��104kJ��1mol������ȫȼ�շ���2900kJ������ȼ���ȸ���д���Ȼ�ѧ����ʽ��C4H10(g)+![]() O2(g)=4CO2(g)+H2O(l)��H= -2900kJ/mol��
O2(g)=4CO2(g)+H2O(l)��H= -2900kJ/mol��
(2)��H2(g)��ȼ������HΪ-285.8kJmol-1֪��1mol H2(g)��ȫȼ������1mol H2O(l)�ų�����285.8kJ�����ֽ�1mol H2O(l)Ϊ1mol H2(g)���ĵ�����Ϊ285.8kJ����ֽ�10mol H2O(l)���ĵ�����Ϊ285.8kJ��10=2858kJ��
����CO(g)��CH3OH(l)��ȼ������H�ֱ�Ϊ-283.0kJmol-1��-726.5kJmol-1����CO(g)+![]() O2(g)=CO2(g)��H= -283.0kJmol-1����CH3OH(l)+
O2(g)=CO2(g)��H= -283.0kJmol-1����CH3OH(l)+![]() O2(g)=CO2(g)+2 H2O(l)��H= -726.5kJmol-1���ɸ�˹���ɿ�֪�â�-�ٵ÷�ӦCH3OH(l)+O2(g)=CO(g)+2 H2O(l)���÷�Ӧ�ķ�Ӧ����H= -726.5kJmol-1-(-283.0kJmol-1)= -443.5kJmol-1��
O2(g)=CO2(g)+2 H2O(l)��H= -726.5kJmol-1���ɸ�˹���ɿ�֪�â�-�ٵ÷�ӦCH3OH(l)+O2(g)=CO(g)+2 H2O(l)���÷�Ӧ�ķ�Ӧ����H= -726.5kJmol-1-(-283.0kJmol-1)= -443.5kJmol-1��
����֪����2SO2(g)+O2(g)![]() 2SO3(g)��H= -196.6kjmol-1����2NO(g)+O2(g)
2SO3(g)��H= -196.6kjmol-1����2NO(g)+O2(g)![]() 2NO2(g)��H= -113.0kJmol-1�����ݸ�˹���ɼ���
2NO2(g)��H= -113.0kJmol-1�����ݸ�˹���ɼ���![]() �ɵ�NO2(g)+SO2(g)
�ɵ�NO2(g)+SO2(g)![]() SO3(g)+NO(g)����H=
SO3(g)+NO(g)����H=![]() = -41.8kJ/mol��
= -41.8kJ/mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ��3D��ӡ�������Ե����̼�������缫���ϣ�������ZnSO4��Һ���л��߾���Ϊ��̬����ʣ������ܷ�ӦΪ��
![]() �����ؽṹ��ͼ1��ʾ��ͼ2���л��߾���ĽṹƬ�Ρ�����˵������ȷ����
�����ؽṹ��ͼ1��ʾ��ͼ2���л��߾���ĽṹƬ�Ρ�����˵������ȷ����
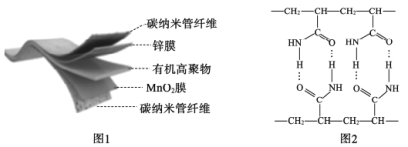
A.�ŵ�ʱ������пĤ��̼������ά����ظ���
B.�л��߾����к��еĻ�ѧ���У����Լ����Ǽ��Լ������
C.�ϳ��л��߾���ĵ�����![]()
D.�ŵ�ʱ���缫��������ӦΪ��MnO2+e-+H2O=MnOOH+OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽��ͬ����ǽ���Ԫ�����ʵĵݱ���ɣ�ij�о���ѧϰС��ļ�ͬѧ�������ͼ��ʾ��ʵ��װ�ã�����Aװ���ڿ�����Cl2��
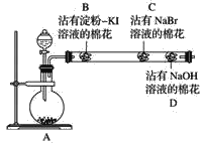
(1)��ͬѧ�IJ���ʵ���¼���£�B���������ɫ��C������ɳȺ�ɫ����ͬѧ�ɴ�����ó��Ľ�����ͬ������ϵ���Ԫ�صķǽ����Լ�����
��B��������Ӧ�Ļ�ѧ����ʽΪ_________��
�ڼ�ͬѧ�Ľ�����________(������ȷ����������ȷ��)�ģ�������____________________��
(2)��ͬѧ��ΪӦ����ͬѧʵ����B��C������������λ�ã����������û����壬Ȼ��������KI�Ӵ������ж�Br2��KI�ܷ�����Ӧ����������________(����������������������)�ģ�ԭ����___
(3)��ͬѧ�ڼ�ͬѧ��ʵ�������ȡ��C����������������һ��մ�е���KI��Һ����(E)�Ӵ�������E����������ɫ����ϼ�ʵ����������Ϊ����ȷ��ͬ����Ԫ�طǽ����Եĵݱ���ɣ���ͬ����ֹ۵���______(����ͬ����������ͬ����)��������__________
(4)��ͬѧ�ۺϷ�����ǰ�漸λͬѧ��ʵ�飬��Ϊ����մ��Na2S��Һ�������ڲ��������ʵ���λ�ã���ʵ�黹����ͬʱ̽��ͬ����Ԫ�����ʵĵݱ���ɣ�������˹۵��������___��Ԥ�ڵ������������___________����Ӧ��Ӧ�����ӷ���ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ú����ȼú��������Ҫ��Ⱦ���Ҫ�ɷ���Al2O3��Fe2O3��MgO��SiO2�����ʡ�������粒�����Լ���ȡ��ú����Al2O3����ȡ���Ĺ���������ͼ��
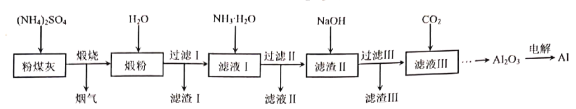
�ش��������⣺
(1)��֪�����к���NH3�����չ�����Al2O3���뷴Ӧ�Ļ�ѧ����ʽΪ___��
(2)������������������������Ҫ�ɷֵĻ�ѧʽ�ֱ�Ϊ__��__��
(3)������Һ����ͨ��__�����ˡ�ϴ�ӡ������õ��ľ���ɷ���__������ѭ�����á�
(4)������Һ������ͨ�����CO2�����ɳ��������ӷ���ʽΪ__��
(5)���Al2O3�Ʊ�Al��Ҫ����ʯ(Na3AlF6)�����ۼ���Na3AlF6����Al(OH)3��Na2CO3��HF��Ӧ�Ƶã���Ӧ�Ļ�ѧ����ʽΪ__��
(6)ij���������������̴�m�ַ�ú�����Ƶ�n��Al2O3����ԭ��ú����������������Ϊ__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25�棬��100mL0.1mol��L-1H2S��Һ�У�ͨ��HCl��������NaOH�����Ե�����ҺpH����ҺpH��c(S2-)��ϵ��ͼ��������Һ����ı仯��H2S�Ļӷ���������˵����ȷ���ǣ� ��
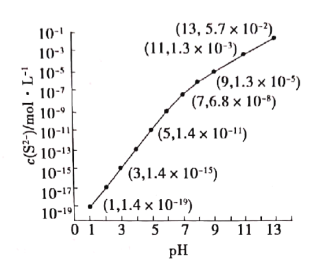
A.��c(H2S)+c(HS-)=0.043mol��L-1ʱ��pH=13
B.pH=1ʱ��c(H+)=c(Cl-)+c(HS-)+ c(S2-)+c(OH-)
C.�����Һ�м���NaOH����������ʱc(Na+)>c(HS-)>c(S2-)>c(H+)=c(OH-)
D.��֪��Ksp(MnS)=2.8��10-13��ij��Һ��amol��L-1Mn2+��0.10mol��L-1H2S������ҺpH=5ʱ��Mn2+��ʼ��������a=0.2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾװ�ÿ�������ȡFe(OH)2�۲�Fe(OH)2�ڿ����б�����ʱ����ɫ�仯��ʵ��ʱ����ʹ����м��6 mol/L H2SO4��Һ��NaOH��Һ����д���пհף�
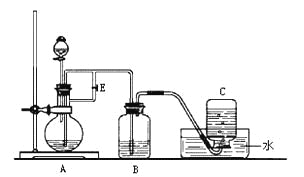
��1��B��ʢ��һ������______________��A��ӦԤ�ȼ�����Լ���_________��
A�з�Ӧ�Ļ�ѧ����ʽΪ________________________________________��
��2��ʵ�鿪ʼʱӦ�Ƚ�����E_______���������������ر�������Ŀ����______________________________________________________________________________��
��3��A��Ӧһ��ʱ�����E_______���������������ر�������Ŀ����____________________________________________________________________________��
��4��ʵ���������ȥװ��B�е���Ƥ����ʹ�������룬Fe(OH)2������������Ӧ������ת����0.04mol���ӣ���μӷ�Ӧ�����������Ϊ____________L����״������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʡ�ī��ֽ���������й���ͳ�Ļ��б���Ϊ���ķ��ı���������˵���д������( )
A.����ʱ�ͷ��ɽ��ë�Ƴɣ������շ��ɼ�������ͻ���
B.������ī����������ȡ���̻��Ƴɣ��ô�īд���վû���ɫ
C.������ֽ��ԭ������̴��Ƥ�͵��ݣ�����Ҫ�ɷ־�����ά��
D.����ʯ��Ҫ�ɷֺ�ˮ����ĸ![]() �������ڹ�����
�������ڹ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ײ���MFe2Ox(3��x��4)��M��ʾ+2�۵Ľ���Ԫ�أ��ڷ�Ӧ�л��ϼ۲������仯�������£�MFe2Ox��ʹ��ҵ�����е�SO2ת��ΪS���������£��������ж���ȷ����( )
![]()
A.MFe2Ox��������
B.SO2�Ǹ÷�Ӧ�Ĵ���
C.x<y
D.MFe2Oy�ǻ�ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȼ�տ���ͨ����������;����
;��I��C3H8(g) + 5O2(g) == 3CO2(g) +4H2O(l) ��H��-a kJ��mol-1
;��II��C3H8(g) ==C3H6(g)+ H2(g) ��H��+b kJ��mol-1
2C3H6(g)+ 9O2(g) == 6CO2(g) +6H2O(l) ��H��-c kJ��mol-1
2H2(g)+O2(g) == 2H2O(l) ��H��-d kJ��mol-1��a��b��c��d��Ϊ��ֵ��
��ش��������⣺
��1���жϵ����ı���ͨ������;���ų���������;��I�ų������� ______��������������������������С������;��II�ų���������
��2������C3H8(g) ==C3H6(g)+ H2(g) �ķ�Ӧ�У���Ӧ����е�������______��������������������������С��������������е�����������ô�ڻ�ѧ��Ӧʱ����Ӧ�����Ҫ______�������ų�������������������������ת��Ϊ���������䷴Ӧ������______________��
��3��b ��a��c��d����ѧ��ϵʽ��______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com