
 O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��
O2(g)===H2O(g)����H����241.8 kJ��mol��1������˵���в���ȷ���� �� ��| A��H2��ȼ����Ϊ241.8 kJ��mol��1 |
| B��2H2(g)��O2(g)===2H2O(g)����H����483.6 kJ��mol��1 |
| C��1 mol H2��ȫȼ������Һ̬ˮ�ų�����������241.8 kJ |
| D���Ͽ�1 mol H2O�Ļ�ѧ�����յ����������ڶ���1 mol H2��0.5 mol O2�Ļ�ѧ�������յ������� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2C(g)�ﵽƽ��ʱ�������ʵ�
2C(g)�ﵽƽ��ʱ�������ʵ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪
����ȼ�ϣ����������������������߷�Ӧ���ɵ�������̬ˮ����֪ ��ȫ����������Ӧ�ų�
��ȫ����������Ӧ�ų� ���������Ȼ�ѧ����ʽ�ǣ�
���������Ȼ�ѧ����ʽ�ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��26.0kJ | B��51.9kJ | C��155.8kJ | D��467.3kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3(g) ��H����93 kJ��mol��1���Ը��ݱ������м������ݣ�����a ����ֵΪ_______kJ��mol��
2NH3(g) ��H����93 kJ��mol��1���Ը��ݱ������м������ݣ�����a ����ֵΪ_______kJ��mol��| ��ѧ�� | H��H | N��H | N��N |
| ����/kJ��mol��1 | 436 | a | 945 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ͬ�¶��£�0.1 mol?L-1NH4Cl��Һ��NH4+��Ũ�ȱ�0.1 mol?L-1��ˮ��NH4+��Ũ�ȴ� |
| B����ϡ����ϴ��AgCl��������ˮϴ�����AgClС�� |
| C����ⱥ��ʳ��ˮʱ�������õ�����������Һ�������� |
D������Al(OH)3(s) Al(OH)3(aq) Al(OH)3(aq) Al3+(aq)+3OH-(aq)��ǰ��Ϊ�ܽ�ƽ�⣬����ǵ� Al3+(aq)+3OH-(aq)��ǰ��Ϊ�ܽ�ƽ�⣬����ǵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CO+3H2���ʱ��H=_______��
CO+3H2���ʱ��H=_______��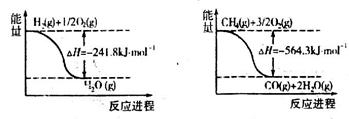
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
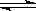 SO3(g) ��H = �D98.32kJ��mol���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ
SO3(g) ��H = �D98.32kJ��mol���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ | A��196.64kJ | B����196.64kJ |
| C��196.64kJ��mol | D����196.64kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
CH3OH(g)��ͼI��ͼ���ǹ��ڸ÷�Ӧ���������ͼʾ��
 ������� ��
������� ��| ���� | �� | �� | �� |
| Ͷ���� | 1mol CO ��2mol H2 | 1molCH3OH | 2molCH3OH |
| CH3OH��Ũ�ȣ�mol��L-1�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�Q1kJ | ����Q2kJ | ����Q3kJ |
| ��ϵѹǿ��Pa�� | P1 | P2 | P3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
 E��2��2=��3
E��2��2=��3�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com