
�����ƹ㷺�����������ȶ��ϳɼ���ӡȾ��ҵ�Ļ�ԭ�������������������շۡ�����ͼ��ᡣ�������õ�ʯ¯������75����90��CO���Լ�����CO2��H2S��N2��CH4��)�ϳɣ���ϳɲ��ֹ����������£�
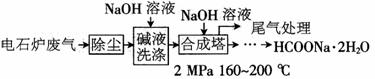
��1�����������ü�Һϴ�ӵ�Ŀ����__________________________________________�����ܷ����ķ�Ӧ��_______________________________________��д������ѧ����ʽ����
��2�������ϳɹ����в���ѭ���������պϳɣ���Ŀ����___________________________�����β����Ҫ�ɷ���__________________��
��3�������Ƹ���ʱ�ֽ���ȡ������(Na2C2O4)�Ļ�ѧ����ʽΪ____________________��
��4���ڼ����ơ��������ƻ����Һ��ͨ������������壮�ɵõ���Ҫ�Ĺ�ҵ��Ʒ���շ�(Na2S2O4)��ͬʱ����������̼���壬�÷�Ӧ�����ӷ���ʽΪ___________________��
��5��ij����֪�ϳ����м�������������Ϊ40%��Ҫ�Ƶ�������������Ϊ5����HCOONa��Һ1�֣���ҪCO�ڱ���µ����Ϊ_______________��
 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڰ����ӵ�������˵����ȷ����(����)
A�������ӵ�������12 g̼��������̼ԭ����
B�������ӵ�������0.012 kg 12C��������ԭ����
C�������ӵ�������6.02��1023
D�������ӵ������ķ���ΪNA������Ϊ6.02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
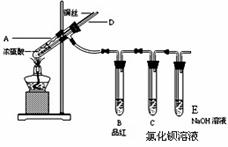 ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ����������ʵ�顣
ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ����������ʵ�顣
ʵ��1��ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��
ʵ�鲽�裺
�������Ӻ�װ�ã����������ԣ������Լ���
�ڼ���A�Թ�ֱ��B��Ʒ����ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿�����뿪Һ�棻
��1���ܹ�֤��ͭ��Ũ���ᷴӦ��������������� ��
��2����ʢ���Ȼ�����Һ��C�Թ��г��˵��ܿ��������⣬���������������������Ϊ���ݣ��ֱ�μ�������Һ�������������Ļ�ѧʽ�����±���Ӧλ�á�
| �μӵ���Һ | ��ˮ | ��ˮ |
| �����Ļ�ѧʽ | __________________________ | __________________________ |
д������SO2��ʾ��ԭ�Ե����ӷ���ʽ�� ��
��3��Ϩ��ƾ��ƺ���Ϊ�е���D�Ĵ��ڣ�B�е�Һ�岻�ᵹ����
��ԭ����: ��
��4����SO2����ͨ�뺬��n mol Na2S��Һ�У���Һ�г��ֻ�ɫ���ǣ��Է�������Һ���������SO2���� mol�� ���������ܽ��SO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧ�Ļ�ѧ֪ʶ���ش��������⣺
(1)�Ӻ�ˮ����ȡþ����Ҫ�����У���Ũ���ᾧ(��HCl������) �ڼ���ʯ�� �ۼ�����
�ܹ��� �����ڵ�⣬�밴��˳��д����ȷ�IJ�������(�����)��____________________
(2) ��ѧ����ʽ(δ��ƽ)As2S3 +H2O+NO3- = AsO43-+NO+ _______+SO42-���ش���������⣺
a. ��Ӧ����Һ��_____��(д�ᡢ���)��
b. ÿ1molˮ�μӷ�Ӧ���÷�Ӧת�Ƶ��ӵ���ĿΪ_______
(3) �������FeSO4��Һ�м��뼸��NaClO��Һ�����Ҽ�������ϡ���ᣬ������Һ��ɻ�ɫ��ijͬѧ��Ϊ�÷�Ӧ�Ļ�ԭ���������ֿ����ԣ�������Cl2��Cl-����ʵ��֤����ԭ����ֻ����Cl- ���ش��������⣺
a. ��ԭ�����в�����������ԭ����__________________________ (�û�ѧ�����ʾ)
b. д���������̵����ӷ���ʽ____________________________________
c. Ϊ�˼���������Ӧ�Ƿ�������Cl-���������ѡ����ʵ���ţ��������Ⱥ�˳�����У�����ʵ�����������ȷ�IJ���˳��Ϊ__________________
A. ȡһ֧�Թܣ�����������Ӧ�����Һ��������B. ����������Ȼ�����Һ��C. ������������ᱵ��Һ�� D. ���������ữ����������Һ���۲쵽��ɫ������E. ���ˣ�ȡ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
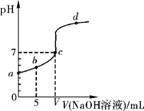
��25���£���10.00mL0.01mol��L-1ijһԪ��HA��Һ����μ���0.01mol.L-1NaOH��Һ����ҺpH�仯��������ͼ��ʾ������˵����ȷ����
A��HA��ǿ��
B��b���ʾ����Һ�У�c(HA) = c(A-)
C��c��ʱ��V=10.00mL
D��b��c��d�����ʾ����Һ��һ�������ڣ�
c(Na+)+c(H+) = c(A-)+c(OH-)
1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ����һС����Ͷ����з�̪��ˮ�У���ʵ����֤���ƾ������������е�(����)
�����ܶȱ�ˮС�����Ƶ��۵�ϵ͡�������ˮ��ӦʱҪ�ų�������������ˮ��Ӧ����Һ�ʼ���
A���٢� B���٢ڢ�
C���٢ۢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4.6 g������Ͷ�뵽95.6 gˮ�У��õ�����Һ�����ʵ�����������
(����)
A��4.6% B��7.6%
C��8% D��10%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£�A�ǿ�����������ˮ���������Ļ���ɫ�������壬A��B��C��D��E����XԪ�أ���ת����ϵ��ͼ��ʾ��
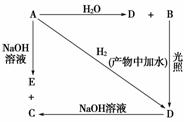
(1)��ֱ�д��A��B��C��D��E�Ļ�ѧʽ(��Ϊ��Һ�������ʵĻ�ѧʽ)��
A________��B________��C________��D__________________________��
E________��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ��
A��H2O______________________________________________________��
A��NaOH___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɫ��ѧ����ָ�Ӽ�������������ƿ��еĻ�ѧ��Ӧ�������ܼ��ٶԻ����ĸ����á����л�ѧ��Ӧ����������ɫ��ѧ������� ( )
A���������᳧β���ŷţ�SO2+2NH3+H2O==(NH4)2SO3
B�����������Ṥҵβ���ĵ���������Ⱦ��NO2+NO+2NaOH==2NaNO2+H2O
C����CuSO4��2Cu+O2==2CuO��CuO+H2SO4==CuSO4+H2O
D����Cu(NO3)2��Cu+4HNO3(Ũ)== Cu(NO3)2+2NO2��+2H2O
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com