
�о�NOx��SO2��CO�ȴ�����Ⱦ����Ĵ�������������Ҫ���塣
��1��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ���������ת��Ϊ����S����֪��
��CO(g)+ O2(g)====CO2(g) ��H=-283.0 kJ��mol-1
O2(g)====CO2(g) ��H=-283.0 kJ��mol-1
��S(s)+ O2(g)==== SO2(g) ��H=-296.0 kJ��mol-1
�˷�Ӧ���Ȼ�ѧ����ʽ��_________________________��
��2��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣
��֪����CO(g)+NO2(g)====NO(g)+CO2(g) ��H=-akJ��mol-1(a>0)
��2CO(g)+2NO(g)= ===N2(g)+
===N2(g)+ 2C
2C O2(g) ��H=-bkJ��
O2(g) ��H=-bkJ�� mol-1(b>0)
mol-1(b>0)
���ñ�״����3.36 L CO��ԭNO2��N2(CO��ȫ��Ӧ)������������ת�Ƶ��ӵ����ʵ���Ϊ________mol���ų�������Ϊ________kJ(�ú���a��b�Ĵ���ʽ��ʾ)��
��3����CH4����ԭNOxҲ�������������������Ⱦ�����磺
��CH4(g)+4NO2(g)====4NO(g)+CO2(g)+2H2O(g) ��H1=-574 kJ��mol-1��
��CH4(g)+4NO(g )====2N2(g)+CO2(g)+2H2O(g)����H2=����
)====2N2(g)+CO2(g)+2H2O(g)����H2=����
��1 mol CH4��ԭNO2��N2���������зų�������Ϊ867 kJ����H2=________��
����������1���������֪��CO��SO2��Ӧ����CO2�͵���S�����ݸ�˹���ɣ��١�2-�ڵã�
2CO(g)+SO2(g)====S(s)+2CO2(g) ��H1=-270 kJ��mol-1
��2���ɢ١���������ʽ��֪��CO�����������ΪCO2��̼Ԫ�صĻ��ϼ۴�+2������+4�ۣ�3.36 L CO��0.15 mol�����ݵ��ӵ�ʧ�غ㣺����ת�Ƶ����ʵ���Ϊ0.3 mol��
���ݸ�˹���ɣ��١�2+�ڵã�
4CO(g)+2NO2(g)====N2(g)+4CO2(g) ��H=-(2a+b)kJ��mol-1
��ų�������Ϊ0.15(2a+ b)/4 kJ=3(2a+b)/80 kJ
��3�����ݸ�˹���ɢ�+�ڿɵã�
2CH4(g)+4NO2(g)====2N2(g)+2CO2(g)+4H2O(g) ��H=(��H1+��H2)
��(��H1+��H2)/2=-867 kJ��mol-1����֮��
��H2= -1 160 kJ��mol-1��
�𰸣���1��2CO(g)+SO2(g)====S(s)+2CO2(g)
��H1=-270 kJ��mol-1
��2��0.3��3(2a+b)/80 ��3��-1 160 kJ��mol-1



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й��������жϻ��ʾ������ȷ����(����)
A����C(s��ʯī)===C(s�����ʯ)����H����1.9 kJ·mol��1����֪��ʯī�Ƚ��ʯ���ȶ�
B�����������������������ֱ���ȫȼ�գ����߷ų�����������
C����H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ·mol��1����֪����1 mol CH3COOH����Һ�뺬1 mol NaOH����Һ��ϣ��ų�����������57.3 kJ
D��2 g H2��ȫȼ������Һ̬ˮ�ų�285.8 kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)��O2(g)===2H2O(l)����H����285.8 kJ·mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������з����ȳ������ܽ�������ǣ� ��
����̼������Һ��ͨ�������CO2 ����Fe(OH)3��������εμ������H2SO4
����Ba(NO3)2��Һ��ͨ�����SO2 ����ʯ��ˮ��ͨ�����CO2
�����������Һ����μ������������
A. �٢ڢ� B.�ڢۢ� C.�ڢ� D. �٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С����������װ��̽�������백��֮��ķ�Ӧ������A��F�ֱ�Ϊ�����������ķ���װ�ã�CΪ�����������백����Ӧ��װ�á�
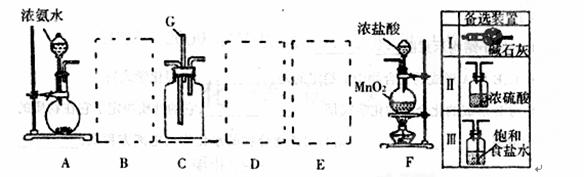
��ش��������⣺
��1��װ��F�з�����Ӧ�����ӷ���ʽ��________________________________________________��
��2��װ��A����ƿ�ڹ������ѡ��__________��ѡ������ѡ��Ĵ��ţ�˫ѡ��2�֣���
A����ʯ�� B����ʯ�� C���������� D���Ȼ��ơ�
��3����ͼA��F�ǰ���ȷʵ��װ��˳�����еġ����߿���Ӧ���ӱ�Ҫ�ij���װ�ã������ͼ�ı�ѡװ����ѡ��������������пո�B__________��D__________��E__________��
��4��Cװ�õķ�Ӧԭ����Ӧ���ڼ�����������Ĺܵ��Ƿ�©����д��������������Ĺܵ��Ƿ�©���Ļ�ѧ����ʽ__________________________________________________________________��
�����һ��ʵ�鷽������ ��______________________________________________________������װ��C��G���ݳ���β���к�������Cl2��д��β�����������ӷ���ʽ�� __________________________________________________________________________________��
��______________________________________________________������װ��C��G���ݳ���β���к�������Cl2��д��β�����������ӷ���ʽ�� __________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݵ���������Ӧ���Ȼ�ѧ����ʽ��
(��)I2(g)+H2(g) 2HI(g) ��H=-9.48 kJ��mol-1
2HI(g) ��H=-9.48 kJ��mol-1
(��)I2(s)+H2(g) 2HI(g) ��H=+26.48 kJ��mol-1
2HI(g) ��H=+26.48 kJ��mol-1
�����ж���ȷ���ǣ� ��
A.254 g I2(g)��ͨ��2 g H2(g)����Ӧ����9.48 kJ
B.1 mol��̬����1 mol��̬���������������17.00 kJ
C.��Ӧ(��)�IJ���ȷ�Ӧ(��)�IJ����ȶ�
D.��Ӧ(��)�ķ�Ӧ���������ȷ�Ӧ(��)�ķ�Ӧ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��R��T��Q��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������T������������������������ȡ������жϲ���ȷ����(����)

A�������̬�⻯������ȶ��ԣ�R��Q
B������������Ӧˮ��������ԣ�Q<W
C��ԭ�Ӱ뾶��T>Q>R
D����T������Һһ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ʾ������Ԫ���У�W��X��Y��ZΪ������Ԫ�أ�������Ԫ�ص�ԭ������������֮��Ϊ22������˵����ȷ����(����)
| X | Y | ||
| W | Z | ||
| T |
A.X��Y��Z����Ԫ����ͼ��⻯��ķе���������
B����X��Y��������Ԫ���γɵĻ�������ֻ�й��ۼ�
C������WY2��W3X4��WZ4�����۵�ߡ�Ӳ�ȴ������
D��TԪ�صĵ��ʾ��а뵼������ԣ�T��ZԪ�ؿ��γɻ�����TZ4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鲻�ܴﵽԤ��Ŀ�ĵ���(����)
| ��� | ʵ����� | ʵ��Ŀ�� |
| A | Cl2��Br2�ֱ���H2��Ӧ | �Ƚ��ȡ���ķǽ�����ǿ�� |
| B | MgCl2��AlCl3��Һ�зֱ�ͨ��NH3 | �Ƚ�þ�����Ľ�����ǿ�� |
| C | �ⶨͬŨ�ȵ�Na2CO3��Na2SO4��Һ��pH | �Ƚ�̼����ķǽ�����ǿ�� |
| D | Fe��Cu�ֱ������ᷴӦ | �Ƚ�����ͭ�Ľ�����ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�й��Ȼ�ѧ����ʽ��д���Ӧ��������ȷ���� (����)
A��ϡ������0.1 mol·L��1 NaOH��Һ��Ӧ��H��(aq)��OH��(aq)===H2O(l)����H����57.3 kJ·mol��1
B���ܱ������У�9.6 g�����11.2 g���ۻ�ϼ�������������17.6 gʱ���ų�19.12 kJ��������Fe(s)��S(s)===FeS(s)����H����95.6 kJ·mol��1
C��������ȼ����Ϊ285.5 kJ·mol��1����ˮ�����Ȼ�ѧ����ʽΪ2H2O(l)===2H2(g)��O2(g)����H����28 5.5 kJ·mol��1
5.5 kJ·mol��1
D����֪2C(s)��O2(g)===2CO(g )����H����221 kJ·mol��1�����֪C��ȼ����Ϊ110.5 kJ·mol
)����H����221 kJ·mol��1�����֪C��ȼ����Ϊ110.5 kJ·mol ��1
��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com