
| 1000��w |
| M |
| n |
| V |
| 1000��1.84��98% |
| 98 |
| 1mol/L��0.1L |
| 18.4mol/l |


 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ȶ��ԣ�Na2CO3��NaHCO3 |
| B����ͬ�����£�����ˮ��Һ�ļ��ԣ�Na2CO3��NaHCO3 |
| C�������£���ˮ�е��ܽ�ȣ�Na2CO3��NaHCO3 |
| D����ͬ�����£���ϡ���ᷴӦ�Ŀ�����Na2CO3��NaHCO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
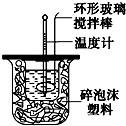 ��ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
��ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͼ��ÿһ�����ʾ�йص�һ�ַ�Ӧ����������֪B��һ�ֵ��ʣ��������ʶ��Ǻ���BԪ�صĻ����C��һ�����Σ�E��C��Ӧ���B��D�ľ��嶼�Ǹ��۵㡢��Ӳ�Ĺ��壬��DΪB���������������BԪ�صķ�Ӧ���Լ�������Ӧ���ӵı�Ҫ�Լ��ͷ�Ӧ����������ȥ����
ͼ��ÿһ�����ʾ�йص�һ�ַ�Ӧ����������֪B��һ�ֵ��ʣ��������ʶ��Ǻ���BԪ�صĻ����C��һ�����Σ�E��C��Ӧ���B��D�ľ��嶼�Ǹ��۵㡢��Ӳ�Ĺ��壬��DΪB���������������BԪ�صķ�Ӧ���Լ�������Ӧ���ӵı�Ҫ�Լ��ͷ�Ӧ����������ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
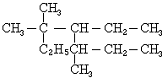 A��ij����2���������ӳɺ�õ�2��2-�������飬��ϵͳ������������������
A��ij����2���������ӳɺ�õ�2��2-�������飬��ϵͳ�������������������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com