
1��2������������������������Ӽ���������������ɫҺ�壬�ܶ�2.18 g��cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�����ͼ��ʾ�豸�Ʊ�1��2���������飮ͼ�з�Һ©������ƿa��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d��װ��Һ�� (���渲������ˮ)��
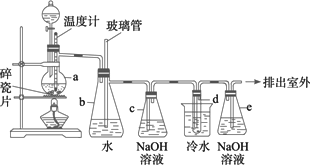
��д���пհף�
(1)��ȫƿb���Է�ֹ���������ɼ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����________��
(2)����c�е�NaOH��Һ��������________��
|
�����𰸣�(1)�������е�Һ��ͻ����� ����(2)��ȥ�������������Ͷ�����̼ ����������ʵ������ȡ��ϩ�ķ���ʽΪCH3CH2OH ����װ���а�ȫƿb���Է�ֹ������Ҳ�ܼ��ʵ�����ʱ�Թ�d�Ƿ���������ԭ�����£� ����������ȫƿ��ѹ��С�����ͨ�������ܴ�����������壬��������ѹǿ��ȣ��Ӷ���ֹ������ ���������Թ�d������������ȫƿ�ڲ�ѹǿ���������е�Һ��ͻ������� |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2012��߶���һ���¿���ѧ���� ���ͣ�058
1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18 g��cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������飮���з�Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ��(���渲������ˮ)����д���пհף�

(1)д���������Ʊ�1��2�����������������ѧ��Ӧ����ʽ________��________
(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����________��
(3)����c��NaOH��Һ��������________��
(4)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������������³������࣮���װ�õ�������û�����⣬���ܵ�ԭ���Т���ϩ����(��ͨ��Һ��)���ʹ��죬��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���߿������С�����ѧ ���ͣ�043
1��2����������������Ϳ����������Ӽ��������£�������ɫҺ�壬�ܶ�Ϊ2.18g��cm��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������飮ͼ�У���Һ©������ƿa��װ���Ҵ���Ũ����Ļ��Һ���Թ�d��װ��Һ��(���渲������ˮ)��

(1)д���������Ʊ�1��2�����������������ѧ��Ӧ����ʽ��________��
(2)��ȫƿb���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d�Ƿ�����������д����������ʱƿb�е�����________��
(3)����c��NaOH��Һ��������________��
(4)ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ��������������³������࣮���װ�õ�������û�����⣬�Է�������ܵ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18
g?��3���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾ�Ʊ�1��2���������顣���з�Һ©������ƿa ��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d ��װ��Һ�壨���渲������ˮ����

��д���пհף�
��д���������Ʊ�1��2�����������������ѧ����ʽ ��
��
�ư�ȫƿb ���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d �Ƿ�����������д����������ʱƿb �е����� ��
������c ��NaOH��Һ�������� ��
��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���ŨH2SO4���Һ����������������������ࡣ���װ�õ�������û�����⣬�Է�������ܵ�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����5�֣�
��ͼ�е�װ�ÿ�������ȡ��Ȳ����ش�ͼ��A�ܵ�����_______________
��ȡ��Ȳ�ķ���ʽ�ǣ�___________________________��
Ϊ���ⷴӦ̫Ѹ�٣��ɲ�ȡ�Ĵ�ʩ��______________________��

��2����7�֣�1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g・mL���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������顣���з�Һ©������ƿa ��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d ��װ��Һ�壨���渲������ˮ����

��д���пհף�
��д���������Ʊ�1��2�����������������ѧ����ʽ��_________________________ �� __________________________ ��
�ư�ȫƿb ���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d �Ƿ�����������д����������ʱƿb �е���__________________________
������c ��NaOH��Һ�������� ________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����5�֣�
��ͼ�е�װ�ÿ�������ȡ��Ȳ����ش�ͼ��A�ܵ�����_______________
��ȡ��Ȳ�ķ���ʽ�ǣ�___________________________��
Ϊ���ⷴӦ̫Ѹ�٣��ɲ�ȡ�Ĵ�ʩ��______________________��

��2����7�֣�1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g・mL���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������顣���з�Һ©������ƿa ��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d ��װ��Һ�壨���渲������ˮ����

��д���пհף�
��д���������Ʊ�1��2�����������������ѧ����ʽ��_________________________ �� __________________________ ��
�ư�ȫƿb ���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d �Ƿ�����������д����������ʱƿb �е���__________________________
������c ��NaOH��Һ�������� ________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com