
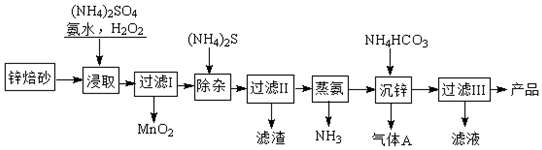
���� �Ʊ�2Zn��OH��2•ZnCO3����Ϊ��п��ɰ����Ҫ�ɷ�ΪZnO��������Cu2+��Mn2+�����ӣ��м�������李���ˮ��˫��ˮ��˫��ˮ�������������ɶ������̣����˺�����Һ�м���泥��ٹ��ˣ���ȥͭ���ӣ�������ȥ����İ���������̼����淋õ�2Zn��OH��2•ZnCO3�Ͷ�����̼���壬���˵�2Zn��OH��2•ZnCO3����ҺΪ�������Һ��
��1����NH4��2SO4��NH3•H2O�Ļ����Һ�д��ڵ���غ㣺c ��NH4+��+c��H+��=2c��SO42-��+c��OH-�����ٽ��c��NH4+��=2c��SO42-���ж���Һ����ԣ�
��2������Ӱ�췴Ӧ���ʵ������жϡ���ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩ��
��3������ȡ��ʱ�����NH3•H2O��������Һ�ʼ��ԣ�˫��ˮ��������������MnO2�����ݵ���غ��Ԫ���غ���д���ӷ���ʽ��
��4�����ݷ�ӦZnS+Cu2+=CuS+Zn2+����֪K=$\frac{{K}_{sp}��ZnS��}{{K}_{sp}��CuS��}$=1.2��1012�����������Ϣ���ж�ZnS���ӣ��Ƿ���У�
��5������п���Ĺ���Ϊ��Һ�е�п������̼�������Һ��Ӧ����2Zn��OH��2•ZnCO3���ݴ���д���ӷ���ʽ��
��6��������3��������ҺΪ�������Һ����ѭ��ʹ�ã�
��� �⣺�Ʊ�2Zn��OH��2•ZnCO3����Ϊ��п��ɰ����Ҫ�ɷ�ΪZnO��������Cu2+��Mn2+�����ӣ��м�������李���ˮ��˫��ˮ��˫��ˮ�������������ɶ������̣����˺�����Һ�м���泥��ٹ��ˣ���ȥͭ���ӣ�������ȥ����İ���������̼����淋õ�2Zn��OH��2•ZnCO3�Ͷ�����̼���壬���˵�2Zn��OH��2•ZnCO3����ҺΪ�������Һ��
��1��NH4��2SO4��NH3•H2O�Ļ����Һ�д��ڵ���غ㣺c ��NH4+��+c��H+��=2c��SO42-��+c��OH-������c��NH4+��=2c��SO42-��ʱ��c��H+��=c��OH-��������Һ�����ԣ�
�ʴ�Ϊ���У�
��2������Ӱ�췴Ӧ���ʵ������жϡ���ȡ��ʱΪ�����п�Ľ����ʣ��ɲ�ȡ�Ĵ�ʩΪ���衢�ʵ����ȣ�
�ʴ�Ϊ�����衢�ʵ����ȣ�
��3������ȡ��ʱ�����NH3•H2O��������Һ�ʼ��ԣ�˫��ˮ��������������MnO2����Ӧ�����ӷ���ʽΪMn2++H2O2+2NH3•H2O=MnO2��+2NH4++2H2O��
�ʴ�Ϊ��Mn2++H2O2+2NH3•H2O=MnO2��+2NH4++2H2O��
��4�����ݷ�ӦZnS+Cu2+=CuS+Zn2+����֪K=$\frac{{K}_{sp}��ZnS��}{{K}_{sp}��CuS��}$=1.2��1012����1��105��K��105��ѧ��Ӧ��ȫ������ѡ��ZnS���г����ǿ��еģ�
�ʴ�Ϊ�����У�ZnS+Cu2+=CuS+Zn2+ K=$\frac{{K}_{sp}��ZnS��}{{K}_{sp}��CuS��}$=1.2��1012����1��105��
��5������п���Ĺ���Ϊ��Һ�е�п������̼�������Һ��Ӧ����2Zn��OH��2•ZnCO3�����ӷ���ʽΪ3Zn2++6HCO3-=2Zn��OH��2•ZnCO3��+5CO2��+H2O��
�ʴ�Ϊ��3Zn2++6HCO3-=2Zn��OH��2•ZnCO3��+5CO2��+H2O��
��6��������3��������ҺΪ�������Һ����ѭ��ʹ�ã��仯ѧʽΪ��NH4��2SO4��
�ʴ�Ϊ����NH4��2SO4��
���� ���⿼���������Ʊ���������ơ����ʷ������ᴿ�������ۺ�Ӧ�ã���Ŀ�ѶȽϴ���ȷ�Ʊ�����Ϊ���ؼ���ע�����ճ������ʷ������ᴿ�IJ�������������֪ʶ��϶ࡢ�ۺ��Խ�ǿ����ֿ�����ѧ���ķ�����������������ѧʵ��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� | HClO4 | HCl | HNO3 |
| Ka | 1.6��10-5 | 1.6��10-9 | 4.2��10-10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ��Ͷ��һС������� | B�� | ��ˮ������� | ||
| C�� | ��ˮ��ͨ��SO2 | D�� | ��ˮ�м���NaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Ȼ�� | B�� | Һ���������� | C�� | ̫���ܡ����� | D�� | ȼú��97#���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڢޢ� | B�� | �٢ڢ� | C�� | �ۢߢ� | D�� | �٢ݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3��Һ��HCO3-+H2O?CO32-+H3O+ | |
| B�� | NaHS��Һ��HS-+H2O?H2S+OH- | |
| C�� | ��������Һ��̼��������Һ��Ӧ��Al3++3HCO3-+6H2O?Al��OH��3��+3CO2�� | |
| D�� | NH4Cl����D2O��NH4++D2O?NH3HDO+H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ħ������Ϊ16�� | |
| B�� | ��״���£�0.3molSO2�к���ԭ����Ϊ0.3NA | |
| C�� | �����£�9.5��MgCl2�����к�Mg2+Ϊ0.1 NA | |
| D�� | ��״���£�22.4L H2O��10 NA������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol H2O��������������Ŀһ����NA | |
| B�� | 1 molNH4+��������������10NA | |
| C�� | 51 g NH3����ԭ����Ϊ3NA | |
| D�� | ��������Է���������2NA����ԭ����������gΪ��λ������ֵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥNH4Cl��Һ�е�FeCl3��������Һ�м��백ˮ����pH | |
| B�� | ��ȥ��������Cu2+��Hg2+��������Һ�м���H2S��Na2S�ȳ����� | |
| C�� | ��ȥij��Һ�е�SO42-������Һ�м���þ�� | |
| D�� | ��ȥZnCl2��Һ�е�Fe3+������Һ�м���ZnO |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com