
| �� | �� | �� |
| ���� | �ƹ� | ��� |
| HOCH2COOH | C3H4O5 | 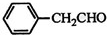 |
 $\stackrel{Fe��HCl}{��}$
$\stackrel{Fe��HCl}{��}$ $��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$
$��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$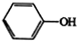
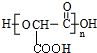 +��n-1��H2O��
+��n-1��H2O��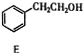 $\stackrel{������}{��}$
$\stackrel{������}{��}$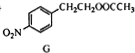 $��_{��2��H+����}^{��1��Fe��HCl}$$\stackrel{{C}_{8}{H}_{11}NO}{H}$$��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$J
$��_{��2��H+����}^{��1��Fe��HCl}$$\stackrel{{C}_{8}{H}_{11}NO}{H}$$��_{��2��H_{2}O��H+����}^{��1��NaNO_{2}��HCl}$J���� ��1���ɼ�����֪�ٵõ�������A�ķ���ʽ��B�Ľṹ��֪��A���巢��ȡ����Ӧ����B����AΪCH3COOH��B�ڼ��������·���ˮ�ⷴӦ���кͷ�Ӧ�õ�DΪHOCH2COONa��D�ữ�õ��ף�
��2��1mol������NaHCO3�����ʵ����Ǽ�2�������ҷ����к���2���Ȼ�������һ��������������״֬���л��߷��ӻ��������ΪHOOCCH��OH��COOH���õ��ĸ߷��ӻ�����Ϊ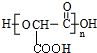 ��
��
��3��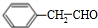 �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ����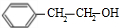 ��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ
��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ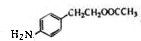 ��JΪ
��JΪ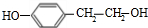 ��
��
��� �⣺��1���ɼ�����֪�ٵõ�������A�ķ���ʽ��B�Ľṹ��֪��A���巢��ȡ����Ӧ����B����AΪCH3COOH��B�ڼ��������·���ˮ�ⷴӦ���кͷ�Ӧ�õ�DΪHOCH2COONa��D�ữ�õ��ף�
�ټ��к��в����ͼ��Ĺ���������Ϊ���Ȼ����ʴ�Ϊ���Ȼ���
��A��BΪȡ����Ӧ��A�Ľṹ��ʽΪ��CH3COOH���ʴ�Ϊ��CH3COOH��
��B��D�Ļ�ѧ����ʽΪ��BrCH2COOH+2NaOH$��_{��}^{H_{2}O}$HOCH2COONa+NaBr+H2O��
�ʴ�Ϊ��BrCH2COOH+2NaOH$��_{��}^{H_{2}O}$HOCH2COONa+NaBr+H2O��
��2��1mol������NaHCO3�����ʵ����Ǽ�2�������ҷ����к���2���Ȼ�������һ��������������״֬���л��߷��ӻ��������ΪHOOCCH��OH��COOH���õ��ĸ߷��ӻ�����Ϊ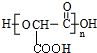 ����Ӧ����ʽΪ��n HOOCCH��OH��COOH$\stackrel{һ������}{��}$
����Ӧ����ʽΪ��n HOOCCH��OH��COOH$\stackrel{һ������}{��}$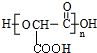 +��n-1��H2O��
+��n-1��H2O��
�ʴ�Ϊ��n HOOCCH��OH��COOH$\stackrel{һ������}{��}$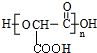 +��n-1��H2O��
+��n-1��H2O��
��3��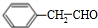 �����������ӳɷ�Ӧ����
�����������ӳɷ�Ӧ����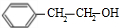 ��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ
��E����������Ӧ��������Ӧ�õ�G��G������Ϣ����ת���õ�HΪ ��JΪ
��JΪ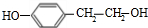 ��
��
���û�ѧ������ȥE�в���������������1�������Լ�������Ϊ����������ͭ��������Һ����������������������������ˮ�ı������Σ���2��3�������ֱ��ǹ��ˡ���Һ���ʴ�Ϊ������������ͭ��������Һ��
�ھ�E��G��H�����Ĺ��������ǻ������Ա����л��������д��ں��ֹ����ŵ������Ǻ�������ǣ�
�ʴ�Ϊ���ǻ�����������ǣ�
��J��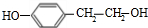 ����ͬ���칹�����ں˴Ź�����������ʾΪ����壬�������Ϊ3��2����״�Ҳ�����֧�����칹�壬�����к���2��-CH3��2��-CH2-����Ϊ�Գƽṹ�����ܵĽṹ��ʽΪ��
����ͬ���칹�����ں˴Ź�����������ʾΪ����壬�������Ϊ3��2����״�Ҳ�����֧�����칹�壬�����к���2��-CH3��2��-CH2-����Ϊ�Գƽṹ�����ܵĽṹ��ʽΪ��
CH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��
CH3CH2OC��C-C��COCH2CH3��CH3OCH2C��C-C��CCH2OCH3��
CH3OC��CCH2-CH2C��COCH3��CH3C��COCH2-CH2OC��CCH3��
CH3C��CCH2OOCH2C��CCH3��CH3CH2C��COOC��CCH2CH3��
����8�֣�����ij�칹��L�еĹ����Ŷ�����H2�����ӳɷ�Ӧ����L�Ľṹ��ʽΪ CH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��
�ʴ�Ϊ��8��CH3CH2COC��CCOCH2CH3��CH3COCH2C��CCH2COCH3��
���� ���⿼���л�����ƶ���ϳɡ������Žṹ���л���Ӧ����ʽ��д����������ͬ���칹�����д�ȣ���3����ͬ���칹����дΪ�״��㡢�ѵ㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������Һ����һ������ˮ��Һ�У��μ�AgNO3������ǡ���ܽ� | |
| B�� | ����Cu��OH��2����Һ����һ����CuSO4��Һ�У���������NaOH��Һ | |
| C�� | 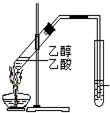 �Ʊ���������������ͼ��ʾ��ʵ��װ�� �Ʊ���������������ͼ��ʾ��ʵ��װ�� | |
| D�� | ����ϩ�ͱ�����������Ȼ�̼��Һ�ֱ�μӵ���������ϩ�ͱ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ɫ��ζ��Һ̬��������ȼ�� | |
| B�� | ����Ũ���ᡢŨ�����Ϲ��ȿ���ȡ������ | |
| C�� | ��ˮ���뱽�����ӳɷ�Ӧ����ɫ | |
| D�� | ��һ�������±���������Ӧ��ȡ�����飬˵�������ӽṹ����̼̼˫�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SeO4�������Ա�Cl2�� | B�� | SeO2�������Ա�SO2�� | ||
| C�� | H2SeO4�������Ա�H2SeO3ǿ | D�� | ŨH2SeO4�������Ա�HNO3ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڵ�ȼ��ȼ����ǰ�������������Ĵ��� | |
| B�� | ��ϡ��Ũ����ʱ��Ӧ��Ũ�����ò�������������ע��ˮ�� | |
| C�� | Ũ�����и�ʴ�ԣ�մ��Ƥ���ϣ�Ӧ�ý϶��ˮ��ϴ����Ϳ��̼������ϡ��Һ | |
| D�� | ���õ�ȼ�����ƾ��ľƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
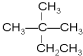 �ܹ��� ��CH3COOH
�ܹ��� ��CH3COOH  ��
��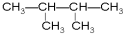 ��
��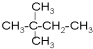 �����
�����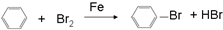 ��
�� ��
��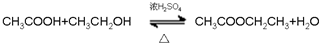 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �Ȼ�����ʹʪ�����ɫʯ����ֽ��Ϊ��ɫ������ΪHCl�����к���H+ | |
| B�� | �Ȼ��⼫������ˮ�����³�ѹ��1���ˮ���ܽ�500���HCl | |
| C�� | �Ȼ�������������ſ������ռ� | |
| D�� | �Ȼ��������Ļ�ѧʽ����HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com