
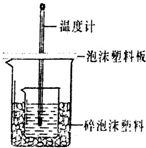
 ijŹµŃ銔×éÉč¼ĘÓĆ0.55mol/LµÄNaOHČÜŅŗ50mLÓė0.50mol/LµÄŃĪĖį50mLÖĆÓŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠ²ā¶ØÖŠŗĶČȵďµŃ飮
ijŹµŃ銔×éÉč¼ĘÓĆ0.55mol/LµÄNaOHČÜŅŗ50mLÓė0.50mol/LµÄŃĪĖį50mLÖĆÓŚČēĶ¼ĖłŹ¾µÄ×°ÖĆÖŠ½ųŠŠ²ā¶ØÖŠŗĶČȵďµŃ飮| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | ĪĀ²ī£Øt2-t1£©”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 28.3 | 3.3 |
| 2 | 25.1 | 25.1 | 25.1 | 28.5 | 3.4 |
| 3 | 25.1 | 25.1 | 25.1 | 28.6 | 3.5 |
| Q |
| n |
| 1.421kJ |
| 0.025mol |


 Č«ÓŵćĮ·µ„ŌŖ¼Ę»®ĻµĮŠ“š°ø
Č«ÓŵćĮ·µ„ŌŖ¼Ę»®ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 ijŹµŃ銔×éÉč¼ĘÓĆ50mL 1.0mol/LŃĪĖįøś50mL 1.1mol/LĒāŃõ»ÆÄĘČÜŅŗŌŚĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®ŌŚ“óÉÕ±µ×²æµęÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½£®Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
ijŹµŃ銔×éÉč¼ĘÓĆ50mL 1.0mol/LŃĪĖįøś50mL 1.1mol/LĒāŃõ»ÆÄĘČÜŅŗŌŚĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦£®ŌŚ“óÉÕ±µ×²æµęÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½£®Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż£®Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ£®ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č£Øt2£©”ę | ĪĀ²ī£Øt2-t1£©”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
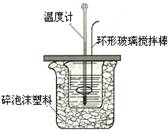 ijŹµŃ銔×éÉč¼ĘÓĆ50 mL1.0mol/LŃĪĖįøś50mL1.1mol/LĒāŃõ»ÆÄĘČÜŅŗŌŚĻĀĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦”£ŌŚ“óÉÕ±µ×²æµęÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½”£Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż”£Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
ijŹµŃ銔×éÉč¼ĘÓĆ50 mL1.0mol/LŃĪĖįøś50mL1.1mol/LĒāŃõ»ÆÄĘČÜŅŗŌŚĻĀĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦”£ŌŚ“óÉÕ±µ×²æµęÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½”£Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż”£Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©±¾ŹµŃéÖŠÓĆÉŌ¹żĮæµÄNaOHµÄŌŅņ½Ģ²ÄÖŠĖµŹĒĪŖ±£Ö¤ŃĪĖįĶźČ«±»ÖŠŗĶ”£ŹŌĪŹ£ŗŃĪĖįŌŚ·“Ó¦ÖŠČōŅņĪŖÓŠ·ÅČČĻÖĻ󣬶ųŌģ³ÉÉŁĮæŃĪĖįŌŚ·“Ó¦ÖŠ»Ó·¢£¬Ōņ²āµĆµÄÖŠŗĶČČŹżÖµ £ØĢīĘ«øß”¢Ę«µĶ»ņ²»±ä£©£»
£Ø2£©øĆŹµŃ銔×é×öĮĖČż“ĪŹµŃ飬Ćæ“ĪČ”ČÜŅŗø÷50mL£¬²¢¼ĒĀ¼ČēĻĀŌŹ¼Źż¾Ż”£
| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č£Øt2£©”ę | ĪĀ²ī£Øt2-t1£©”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
ŅŃÖŖŃĪĖį”¢NaOHČÜŅŗĆÜ¶Č½üĖĘĪŖ1.00g/cm3ÖŠŗĶŗó»ģŗĶŅŗµÄ±ČČČČŻ C=4.18J(g.”¤”ę)ŌņøĆ·“Ó¦µÄÖŠŗĶČČĪŖ”÷H=___________________________________£»
£Ø3£©ČōÓƵČÅØ¶ČµÄ“×ĖįÓėNaOHČÜŅŗ·“Ó¦£¬Ōņ²āµĆµÄÖŠŗĶČȵďżÖµ»į £ØĢīĘ«øß”¢Ę«µĶ»ņ²»±ä£©£¬ĘäŌŅņŹĒ £»
£Ø4£©ŌŚÖŠŗĶČČ²ā¶ØŹµŃéÖŠ“ęŌŚÓĆĖ®Ļ“µÓĪĀ¶Č¼ĘÉĻµÄŃĪĖįČÜŅŗµÄ²½Öč£¬ŹŌ·ÖĪö“Ė²Ł×÷²½Öč¶ŌŹµŃé²ā¶ØÖŠŗĶČȵÄÓ°Ļģ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŹµŃ銔×éÉč¼ĘÓĆ50 mL1.0mol/LŃĪĖįøś50mL1.1mol/LĒāŃõ»ÆÄĘČÜŅŗ½ųŠŠÖŠŗĶ·“Ó¦£¬²ā¶ØÖŠŗĶČČ£¬ÖŠŗĶČČ²ā¶ØŹµŃéµÄ¹Ų¼üŹĒŅŖ±Č½Ļ×¼Č·µŲÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬ĮæČČĘ÷ŅŖ¾”Įæ×öµ½¾ųČČ£»ŌŚĮæČČµÄ¹ż³ĢÖŠŅŖ¾”Įæ±ÜĆāČČĮæµÄÉ¢Ź§£¬±Č½Ļ×¼Č·µŲ²āĮæ³ö·“Ó¦Ē°ŗóČÜŅŗĪĀ¶ČµÄ±ä»Æ”£»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÖŠŗĶČČµÄ²ā¶ØĖłŠčµÄ²£Į§ŅĒĘ÷ÓŠ ”£
£Ø2£©±¾ŹµŃéÖŠÓĆÉŌ¹żĮæµÄNaOHµÄŌŅņŹĒ ”£
£Ø3£©ŌŚ·“Ó¦ÖŠČōŅņĪŖÓŠ·ÅČČĻÖĻ󣬶ųŌģ³ÉÉŁĮæHCl»Ó·¢£¬Ōņ²āµĆµÄÖŠŗĶČČŹżÖµ £ØĢīĘ«øß”¢Ę«µĶ»ņ²»±ä£©”£
£Ø4£©øĆŹµŃ銔×é×öĮĖČż“ĪŹµŃ飬Ćæ“ĪČ”ČÜŅŗø÷50mL£¬²¢¼ĒĀ¼ČēĻĀŌŹ¼Źż¾Ż”£
| ŹµŃéŠņŗÅ w. | ĘšŹ¼ĪĀ¶Čt1/”ę w.w.^w.k.&s.5*u.c.#om | ÖÕÖ¹ĪĀ¶Č£Øt2£©”ę[Ą“Ō“:ѧæĘĶųZXXK][Ą“Ō“:ѧ”£æĘ”£Ķų] | ĪĀ²ī£Øt2-t1£©”ę[Ą“Ō“:Z+xx+k.Com] | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 w.w.^w.k.&s.5*u.c.#om | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 w.w.^w.k.&s.5*u.c.#om | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 w.w.^w.k.&s.5*u.c.#om | 31.9 w.w.^w.k.&s.5*u.c.#om | 6.8 |
ŅŃÖŖŃĪĖį”¢NaOHČÜŅŗĆÜ¶Č½üĖĘĪŖ1.00g/cm3£¬ÖŠŗĶŗó»ģŗĶŅŗµÄ±ČČČČŻ C=4.18J/(g”¤”ę)ŌņøĆ·“Ó¦µÄÖŠŗĶČČĪŖ”÷H=_______________________________”£
£Ø5£©ČōÓƵČÅØ¶ČµÄ“×Ėį“śĢęŃĪĖįÓėNaOHČÜŅŗ·“Ó¦£¬Ōņ²āµĆµÄÖŠŗĶČȵďżÖµ»į £ØĢīĘ«øß”¢Ę«µĶ»ņ²»±ä£©£¬ĘäŌŅņ ![]() ”£
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ¼ŖĮÖŹ”øßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
£Ø8·Ö£©Ä³ŹµŃ銔×éÉč¼ĘÓĆ50 mL 1£®0mol/LŃĪĖįøś50mL 1£®1mol/LĒāŃõ»ÆÄĘČÜŅŗŌŚĻĀĶ¼×°ÖĆÖŠ½ųŠŠÖŠŗĶ·“Ó¦”£ŌŚ“óÉÕ±µ×²æµęÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬Ź¹·ÅČėµÄŠ”ÉÕ±±æŚÓė“óÉÕ±±æŚĻąĘ½”£Č»ŗóŌŁŌŚ“󔢊”ÉÕ±Ö®¼äĢīĀśĖéÅŻÄĖÜĮĻ£Ø»ņÖ½Ģõ£©£¬“óÉÕ±ÉĻÓĆÅŻÄĖÜĮĻ°å£Ø»ņÓ²Ö½°å£©×÷øĒ°å£¬ŌŚ°åÖŠ¼äæŖĮ½øöŠ”æ×£¬ÕżŗĆŹ¹ĪĀ¶Č¼ĘŗĶ»·ŠĪ²£Į§½Į°č°ōĶعż”£Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠĖł·Å³öµÄČČĮææɼĘĖćÖŠŗĶČČ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
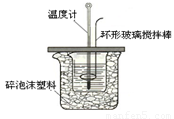
£Ø1£©±¾ŹµŃéÖŠÓĆÉŌ¹żĮæµÄNaOHµÄŌŅņ½Ģ²ÄÖŠĖµŹĒĪŖ±£Ö¤ŃĪĖįĶźČ«±»ÖŠŗĶ”£ŹŌĪŹ£ŗŃĪĖįŌŚ·“Ó¦ÖŠČōŅņĪŖÓŠ·ÅČČĻÖĻ󣬶ųŌģ³ÉÉŁĮæŃĪĖįŌŚ·“Ó¦ÖŠ»Ó·¢£¬Ōņ²āµĆµÄÖŠŗĶČČ
ŹżÖµ £ØĢīĘ«øß”¢Ę«µĶ»ņ²»±ä£©£»
£Ø2£©øĆŹµŃ銔×é×öĮĖČż“ĪŹµŃ飬Ćæ“ĪČ”ČÜŅŗø÷50mL£¬²¢¼ĒĀ¼ČēĻĀŌŹ¼Źż¾Ż”£
|
ŹµŃéŠņŗÅ |
ĘšŹ¼ĪĀ¶Čt1/”ę[Ą“Ō“:Zxxk.Com][Ą“Ō“:Z.xx.k.Com] |
ÖÕÖ¹ĪĀ¶Č£Øt2£©”ę[Ą“Ō“:Z.xx.k.Com] |
ĪĀ²ī£Øt2-t1£©”ę |
||
|
ŃĪĖį |
NaOHČÜŅŗ |
Ę½¾łÖµ |
|||
|
1 |
25£®1 |
24£®9 |
25£®0 |
31£®6 |
6£®6 |
|
2 |
25£®1 |
25£®1 |
25£®1 |
31£®8 |
6£®7 |
|
3 |
25£®1 |
25£®1 |
25£®1 |
31£®9 |
6£®8 |
ŅŃÖŖŃĪĖį”¢NaOHČÜŅŗĆÜ¶Č½üĖĘĪŖ1£®00g/cm3ÖŠŗĶŗó»ģŗĶŅŗµÄ±ČČČČŻ C=4£®18J£Øg£®”¤”ę£©ŌņøĆ·“Ó¦µÄÖŠŗĶČČĪŖ”÷H=___________________________________£»
£Ø3£©ČōÓĆÅØĮņĖįÓėNaOHČÜŅŗ·“Ó¦£¬Ōņ²āµĆµÄÖŠŗĶČȵďżÖµ»į £ØĢīĘ«øß”¢Ę«µĶ»ņ²»±ä£©£¬ĘäŌŅņŹĒ £»
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com