

 D����� E����λ�� F�������� G�����Լ�
D����� E����λ�� F�������� G�����Լ�
| | �۵�/�� | �е�/�� | �ܽ��� |
| ����ȩ | ��26 | 179 | ����ˮ���������Ҵ������� |
| ������ | 122 | 249 | ����ˮ���������Ҵ������� |
| ���״� | ��15.3 | 205.0 | ������ˮ���������Ҵ������� |
| ���� | ��116.2 | 34.5 | ����ˮ���������Ҵ� |
 ��ȡ��Ч�� �� ����á������
��ȡ��Ч�� �� ����á������ ��װ�ã��������������ѣ�Ӧѡ��ļ��ȷ����� �� ������ĸ��
��װ�ã��������������ѣ�Ӧѡ��ļ��ȷ����� �� ������ĸ�� �ţ���
�ţ��� ��ȴʹ�ᾧ��ȫ�����ˣ�ϴ�ӡ����������ϻ���;ֹͣ����ʱ��Ӧ�� �� ��
��ȴʹ�ᾧ��ȫ�����ˣ�ϴ�ӡ����������ϻ���;ֹͣ����ʱ��Ӧ�� �� �� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
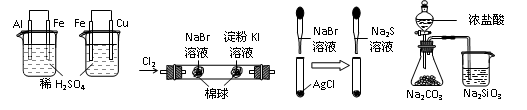
| ʵ �� | ʵ������ | �� �� |
| A | ���ձ��������������ݣ��ұ��ձ���ͭ���������� | ��ԣ�Al��Fe��Cu |
| B | �������Ϊ��ɫ���ұ�����Ϊ��ɫ | �����ԣ�Cl2��Br2��I2 |
| C | ��ɫ�����ȱ�Ϊ����ɫ�����Ϊ��ɫ��Ag2S�� | �ܽ��� ��AgCl��AgBr��Ag2S |
| D | ��ƿ��������������ձ���Һ������ | ���ԣ�HCl��H2CO3��H2SiO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ϡ�������ˣ���ȥ����ͭ���е�����þ�ۺ����� |
| B������ȡ�ķ����������ͺ�ú�� |
| C�����ܽ⡢���˵ķ�������KNO3��NaCl����Ļ���� |
| D����O2��H2�Ļ������ͨ�����ȵ�����ͭ���Գ�ȥ���е�H2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ��һ���Լ���������ᡢ��ȩ������ |
| B������ۺ�ϡ�����ϼ��Ⱥ����Һ�м���������Һ��ˮԡ���ȣ����Լ������ˮ���IJ��� |
| C�����Ҵ���Ũ������170�湲���Ƶõ�����ͨ������KMnO4��Һ�У����Լ������ɵ���ϩ���� |
| D���ñ���Na2CO3��Һ�ռ�������Ҵ���Ӧ���ɵ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
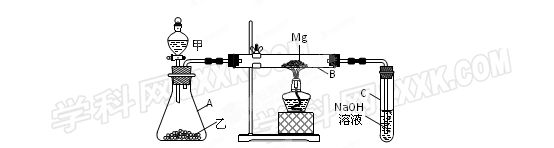 ��1��ѡ����ȡSO2��������Լ� ������ţ���
��1��ѡ����ȡSO2��������Լ� ������ţ���
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ĵ�������ƿ����ˮ���̶��߶��ݣ�����ƿ�����������µߵ�ҡ�ȡ�
�ĵ�������ƿ����ˮ���̶��߶��ݣ�����ƿ�����������µߵ�ҡ�ȡ� ����֮��
����֮�� �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ñ�����KMnO4��Һ�ζ�Na2SO3��Һ�Բ�����Ũ�ȣ�KMnO4�����Ϻ�ɫ |
| B�����á�Ag����SCN��===AgSCN����ԭ�������ñ�KSCN��Һ����AgNO3��ҺŨ�ȣ�Fe(NO3)3������ɫ |
C�����á�2Fe3����2I��===I2��2Fe2������ ��FeCl3��Һ����KI��Ʒ��KI�İٷֺ����� ��FeCl3��Һ����KI��Ʒ��KI�İٷֺ��������ۡ�����ɫ |
| D������OH����H��===H2O������ij������Һ��Ũ��ʱ����̪����dz��ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com