
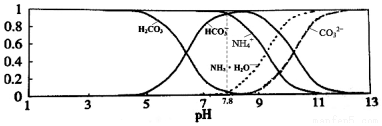
| A�� | ����Һ��pH=9ʱ����Һ�д������й�ϵ��c��NH4+����c��HCO3-����c��NH3•H2O����c��CO32-�� | |
| B�� | ������Һ����εμ���������ʱNH4+��HCO3-Ũ����С | |
| C�� | NH4HCO3��Һ�У�c��NH4+��+c��NH3•H2O��+c��H+��=c��CO32-��+c��H2CO3��+c��HCO3-��+c��OH-�� | |
| D�� | ͨ��������֪������Kb��NH3•H2O����Ka1��H2CO3�� |
���� A����ͼ��֪��pH=9ʱ��c��HCO3-������c��NH4+����
B����̼�������Һ��pH=7.8������ͼ����pH����ʱ��NH4+��HCO3-Ũ�ȱ仯�жϣ�
C������̼�������Һ�е������غ������
D����ͬ�����£����ӵ�ˮ��̶�Խ�����Ӧ��������ʵĵ���ƽ�ⳣ��ԽС��
��� �⣺A�����ͼ���֪����Һ��pH=9ʱ����Һ������Ũ�ȴ�СΪ��c��HCO3-����c��NH4+����c��NH3•H2O����c��CO32-������A����
B��0.1mol/L��NH4HCO3��Һ��pH=7.8������ͼ���֪������ҺpH����ʱ��笠�����Ũ����С����̼����������ܹ���������С����B����
C��NH4HCO3��Һ�д��������غ㣺c��NH4+��+c��NH3•H2O��=c��HCO3-��+c��CO32-��+c��H2CO3������Һ�Լ�����c��H+����c��OH-��������c��NH4+��+c��NH3•H2O��+c��H+����c��CO32-��+c��H2CO3��+c��HCO3-��+c��OH-������C����
D��笠�����ˮ�������ԣ�̼���������ˮ���Լ��ԣ�����0.1mol/L��NH4HCO3��Һ��pH=7.8����Һ�Լ��ԣ�˵��̼��������ӵ�ˮ��̶ȴ���笠����ӵ�ˮ��̶ȣ���Kb��NH3•H2O����Ka1��H2CO3������D��ȷ��
��ѡD��
���� ������ͼ��������Ũ�ȴ�С�Ƚϡ��ε�ˮ��ԭ����֪ʶ����Ŀ�Ѷ��еȣ���ȷͼ�����߱仯�ĺ���Ϊ���ؼ���ע�����յ���غ㡢�����غ㡢�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�÷�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | �ò�˿պȡ��ҺX������ɫ��Ӧʵ�� | ����ʻ�ɫ | ��ҺXһ����������Һ |
| B | ��Cl2ͨ��ʯ����Һ�� | ��Һ�ȱ�����ɫ | Cl2����Ư���� |
| C | ��NaHCO3��Һ�еμ�NaAlO2��Һ | �а�ɫ������������� | AlO2-��HCO3-������˫ˮ�ⷴӦ |
| D | ��FeBr2��Һ�м���������ˮ���ټ� CCl4�� | CCl4����ɫ | Fe2+�Ļ�ԭ��ǿ��Br- |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
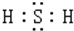 ��H2S��ˮ��Һ�ڿ����з���ʱ��������ǣ���˵����H2S��ǿ�Ļ�ԭ�ԣ�
��H2S��ˮ��Һ�ڿ����з���ʱ��������ǣ���˵����H2S��ǿ�Ļ�ԭ�ԣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
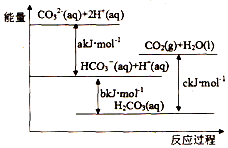
| A�� | H2CO3��aq��=CO2��g��+H2O��l��Ϊ���ȷ�Ӧ | |
| B�� | CO32-��aq��+H+��aq��=HCO3-��aq����H=akJ/mol | |
| C�� | HCO3-��aq��+H+��aq��=CO2��g��+H2O��l����H=��c-b��kJ/mol | |
| D�� | CO32-��aq��+2H+��aq��=CO2��g��+H2O��l����H=��a+b-c��kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
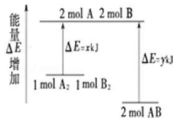
| A�� | �÷�Ӧ�����ȷ�Ӧ | |
| B�� | ���� 1molA-A ���� 1mol B-B ���ų� xkJ ���� | |
| C�� | ���� 2molA-B ����Ҫ���� y kJ ������ | |
| D�� | 1molA2�� 1molB2 ������������ 2molAB �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
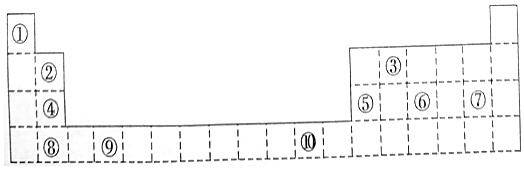
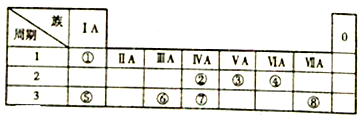
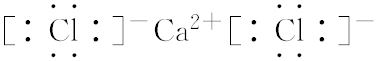 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com