���
�⣺��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ�������ΪK�㡢�����ΪL�㣬���Ԫ��ԭ�Ӻ�����7�����ӣ�ԭ���к��������������ԭ������Ϊ7��������NԪ�أ��ʴ�Ϊ��N��
��2��BԪ�صĸ�һ�����ӵĵ��Ӳ�ṹ�����ͬ����BΪClԪ�أ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�����ͬ����DΪCsԪ�أ�B��C�γɵĻ�����ΪCsCl���������Ӿ��壬������̬�������ƶ������ӣ���������ʱ�ܵ��磬���۷е�ϸߣ��ʴ�Ϊ������ʱ�ܵ��硢�ϸߵ��۵㡢Ӳ�Ƚϴ�ȣ�
��3��DԪ�ص����������ӵ�3d�ܼ�Ϊ���������Dԭ�Ӻ��������=2+8+8+6+2=26��ΪFeԪ�أ���3d��4s����Ϊ��۵��ӣ���۵����Ų�ͼΪ

��
�ʴ�Ϊ��

��
��4���������ڲ���Ԫ��E��F��G�����ݵ�����֪��
EԪ�صڶ��������������ϴ���E�ǵ�IIA��Ԫ�أ�ΪMgԪ�أ�
FԪ�ص������ĵ��������ϴ���F�ǵ�IIIA��Ԫ�أ�ΪAlԪ�أ�
G��һ�������������ϴ���G�ǵ�IA��Ԫ�أ�ΪNaԪ�أ�
F�����������ΪAl
2O
3��G������������Ӧ��ˮ����ΪNaOH�����߷�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽΪAl
2O
3+2OH
-�T2AlO
2-+H
2O��G������������Ӧ��ˮ����NaOHΪ���ӻ����������������Oԭ�Ӻ�Hԭ��֮�乲��һ�Ե��ӣ������ʽΪ
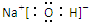
��
�ʴ�Ϊ��Al
2O
3+2OH
-�T2AlO
2-+H
2O��
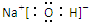
��
��5��1molMg�ĵ�����CO
2������ȼ�շų�760kJ������25�棬101kp
a������2molMg����������������̼��ȼ�շų�1520kJ����2Mg��s��+CO
2��g���T2MgO��s��+C��s����H=-1520 kJ/mol���Ȼ�ѧ����ʽΪ���ʴ�Ϊ��2Mg��s��+CO
2��g���T2MgO��s��+C��s����H=-1520 kJ/mol��

 ��
�� ��
��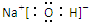 ��
��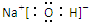 ��
��


