
��10�֣�ʵ���Һϳ����������磨��ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������
��78.5�桢118�桢77.1�棩
��1��������Թ��г��˼����Ҵ��������⣬��Ӧ������Լ���
���Լ��������ǣ���_____ _ __ ��_____________ ��
��2����Ӧ�������Ҳ���Թ�ȡ�£������ã��۲쵽��������
��
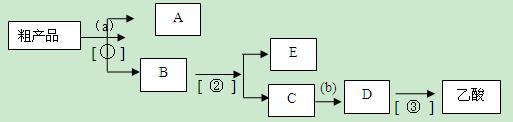
��3��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ���������̣�
����ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a��__________��b��_______________��
���뷽������__________������______________������_______________��
д��C �� D ��Ӧ�Ļ�ѧ����ʽ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩
ʵ���Һϳ����������IJ������£�������ƿ�ڼ����Ҵ���Ũ��������ᣬƿ����ֱ��װͨ����ȴˮ�������ܣ�ʹ��Ӧ��������������ΪҺ��������ƿ�ڣ������Ȼ���һ��ʱ�������װ�ý�����������ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������78.4�桢118�桢77.1�棩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣�ʵ���Һϳ����������磨��ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������
��78.5�桢118�桢77.1�棩
��1��������Թ��г��˼����Ҵ��������⣬��Ӧ������Լ���
���Լ��������ǣ���_____ ___ ��_____________ ��
��2����Ӧ�������Ҳ���Թ�ȡ�£������ã��۲쵽��������
��
��3��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ���������̣�
����ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a��__________��b��_______________��
���뷽������__________������______________������_______________��
д��C �� D ��Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ʡĵ����һ�и�һ��ѧ����ĩ���Ի�ѧ ���ͣ�������
��10�֣�ʵ���Һϳ����������磨��ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������
��78.5�桢118�桢77.1�棩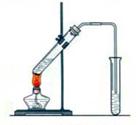
��1��������Թ��г��˼����Ҵ��������⣬��Ӧ������Լ���
���Լ��������ǣ���_____ _ __ ��_____________ ��
��2����Ӧ�������Ҳ���Թ�ȡ�£������ã��۲쵽��������
��
��3��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ���������̣�
����ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a��__________��b��_______________��
���뷽������__________������______________������_______________��
д��C �� D ��Ӧ�Ļ�ѧ����ʽ 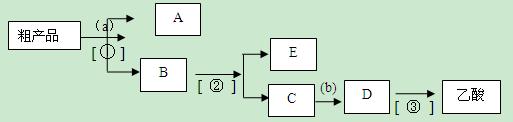
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
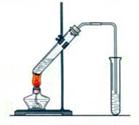
ʵ���Һϳ����������磨��ͼ��ʾ�����õ������Ҵ��������ˮ�����������ֲ�Ʒ����ش��������⣺����֪���Ҵ������ᡢ���������ķе�������
��78.5�桢118�桢77.1�棩
��1��������Թ��г��˼����Ҵ��������⣬��Ӧ������Լ���
���Լ��������ǣ���_____ _ __ ��_____________ ��
��2����Ӧ�������Ҳ���Թ�ȡ�£������ã��۲쵽��������
��
��3��������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ���������̣�
����ͼ��Բ�����������ʵ����Լ����ڷ������������ʵ��ķ��뷽����
�Լ�a��__________��b��_______________��
���뷽������__________������______________������_______________��
д��C �� D ��Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com