
������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᣬ��Ӧԭ
����
ʵ�鷽����һ�����ļױ���������KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
��֪����������Է�������122 ���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3 g��6.9 g�����������л���һ�㶼�й̶��۵㡣
��1��������Ϊ ����Ҫ�õ�����Ҫ��������Ϊ ��������Ϊ ��
��2����ɫҺ��A�� �����Լ���A���Լ��� �������� ��
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ���ͬѧ�Ʋ��ɫ����B�DZ�������KCl�Ļ�����������·��������ᴿ�ͼ��飬ʵ���������Ʋ���ȷ�����ڴ������ɱ������ݡ�
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| �� | ����ɫ����B����ˮ�У� �����ܽ⣬ | �õ���ɫ�������ɫ��Һ | |
| �� | ȡ������Һ���Թ��У� | ���ɰ�ɫ���� | ��Һ��Cl- |
| �� | �����ɫ���壬 | | ��ɫ�����DZ����� |
��1����Һ��1�֣�����Һ©�����ձ��ȣ�1�֣�������1�֣�
��2���ױ���1�֣�������KMnO4��Һ��1�֣�����Һ��ɫ��1�֣���3����� ʵ�鷽�� ʵ������ ���� �� ����ɫ����B����ˮ�м��ȣ��ܽ⣬��ȴ�ᾧ������ ��2�֣� �õ���ɫ�������ɫ��Һ �� ȡ������Һ���Թ��У�����2-3�������ữ��AgNO3��Һ��2�֣� ���ɰ�ɫ���� ��Һ����Cl- �� �����ɫ���壬����ʹ���ڻ��������۵���2�֣� �۵�Ϊ122.4����2�֣� ��ɫ�����DZ�����
��4��(2.40��10-3��122��4)/1.22��2�֣���96%��2�֣�
���������������1��һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�������̷����������ͻ���δ��Ӧ�ļױ������Բ������Ƿ�����л���ױ����÷�Һ�����õ�����Һ��������Ҫ���������Ƿ�Һ©�����ձ��ȣ�������������ķ������Ƽױ��е�õ������ļױ�Һ�壻
��2���������̺��ƶϿ�֪����ɫҺ��AΪ�ױ��������Լ����������Ը��������Һ���ױ�������Ϊ�����ᣬ���������Һ��ɫ��ȥ��
��3��ͨ���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ����ﵽ130��ʱ�����������ۣ��Ʋ��ɫ����B�DZ�������KCl�Ļ����Ȼ��ؿ����������ữ����������Һ���������ӵĴ��ڣ����ñ�������ܽ��������25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g�����ò�ͬ�¶��µ��ܽ�ȣ���������õ������ͨ���ⶨ�۵��ж��Ƿ�Ϊ�����
��4����ȡ1.220g��Ʒ�����100ml�״���Һ����ȡ25.00ml��Һ���ζ�������KOH�����ʵ���Ϊ2.40��10-3mol����������һԪ�������������1��1��Ӧ���������ʵ�����ͬ������Ʒ�б���������������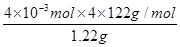 ��100%��96%��
��100%��96%��
���㣺�����������ʵ�ʵ������жϣ����ʷ����������Լ�ѡ�������������Ӧ�ã����ʳɷֵ�ʵ����Ʒ��������衢�Լ�����Ʒ���ȵļ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ijͬѧ������װ���Ʊ�������Cl2�����ʡ�����˵����ȷ����
| A����ͼ�У����MnO2�����������ȫ�������� |
| B����ͼ�У�ʪ�����ɫ������ɫ��֤��Cl2��Ư���� |
| C����ͼ�У�������ɫ�Ĺ��壬֤��Cl2��ǿ������ |
| D����ͼ�У����պ���Ͳ�����������С��˵��������Cl2�����˼ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�������������Ԫ��֮һ������ֲ���纣���������к��зḻ�ġ��Ե�������ʽ���ڵĵ�Ԫ�ء���ʵ�����У��Ӻ�������ȡ������̺�ʵ��װ�����£�
��1��ָ��������ȡ��Ĺ������й�ʵ����������ƣ�
����� ������� ��
��2��д������ܶ�Ӧ��Ӧ�����ӷ���ʽ�� ��
��3����ȡ��Ĺ����У��ɹ�ѡ����л��Լ��� �������ţ�
| A���ƾ� | B������ | C�����Ȼ�̼ | D���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ᣨ�Ҷ��ᣩ������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ����ᣨ��2���ᾧˮ���Ĺ���������ͼ���ش��������⣺
��1��CO��NaOH��һ�������ºϳɼ����ƣ������Ƽ�������Ļ�ѧ��Ӧ����ʽ�ֱ�Ϊ��____��____ ��
��2�����Ʊ������������ι��˲��������˲����ٵ���Һ�� ��������____ �����˲����ڵ���Һ�� �� ��������____ ��
��3�����չ����Тۺܵ͢�Ŀ����____ ��
��4�����˽�������������ֱ���������ữ�Ʊ����ᡣ�÷�����ȱ���Dz�Ʒ���������к��е�������Ҫ��___ ��
��5���ᾧˮ�ϲ����Ʒ�Ĵ����ø�����ط��ⶨ��
���������Ʒ0��250 g����ˮ����0��0500 mol��L-1������KMn04��Һ�ζ�����dz�ۺ�ɫ�����ʣ�����KMn04��Һ15.00 mL����Ӧ�����ӷ���ʽΪ____ ��
��ʽ����ó�Ʒ�Ĵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ����̼���̿�Ϊ��Ҫԭ������MnO2�Ĺ����������£� �й��������↑ʼ�����ͳ�����ȫ��pH���±���
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Al��OH��2 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 | Pb��OH��2 | Mn��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ��ʯ��Ҫ��������ͭ(Cu2S)��������ʯ(SiO2)��һ���Ի�ͭ��ʯΪԭ���Ʊ�����ͭ�Ĺ����������£�
��1��д�����衰��ԭ���п��ܷ�����Ӧ�����ӷ���ʽ �� ��
��2������S�������¶ȿ���50�桫60��֮�䣬���˹�����͵�ԭ���� �� ��
��3������NOx��������Ϻ�ͨ��ˮ�������������п�ѭ�����õ�һ�����ʣ������ʵĻ�ѧʽΪ ������ҺM�м��루��ͨ�룩���� (����ĸ)���ʣ��õ���һ�ֿ�ѭ�����õ����ʡ�
a���� b������ c��������� d������
��4�����³��������м���CuO��Ŀ���� �� ��
��5�����ˢ����õ�����Һ���������������X�����ˢܵõ�����ͭ���塣
����������� ��
����X�� �� (���������) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s)  2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4 ��2H2O
��2H2O
Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
(1)������Ҫ�ɷ���________��________�Լ�δ����±ʯ��
(2)�û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��_________________________________________________��
(3)�����ӡ������У��ȼ���________��Һ��������Ȳ������ˣ��ټ���________��Һ����ҺpH�����ԡ�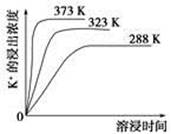
(4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ�
��________________________________________________________��
��________________________________________________________��
(5)�����Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)�� ?
? CaCO3(s)��
CaCO3(s)��
��֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��
Ksp(CaSO4)��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K(������������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�⻯�����Ʊ������л��⻯���ԭ�ϣ���ҽҩ��������̵����������ȡ���ҵ������м��ԭ���Ʊ�NaI������Ҫ��������ͼ�� 
��1��д����мת��ΪFe(OH)3��Ӧ�����ӷ���ʽ�� ��
��2���жϵ�����ȫ��Ӧ�ķ����� ��
��3������Һ�õ�NaI����IJ����� ��
��4���ⶨ��Ʒ��NaI�����ķ����ǣ�
a����ȡ3��000g��Ʒ�ܽ⣬��250mL����ƿ�ж��ݣ�
b����ȡ25��00mL������Һ����ƿ�У�
c����0��100mol ��Һ�ζ����յ㣬����
��Һ�ζ����յ㣬���� ��Һ�����ƽ��ֵΪ19��00mL��
��Һ�����ƽ��ֵΪ19��00mL��
�������ⶨ�������������У���Ҫ����Ƿ�©Һ�������� ��
����ʹ��ǰ�������ϴ�������� ��
��������Ʒ��NaI����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ����������ɫ��ʽ�Ρ������Ե�Ʒ�Һ(��Ҫ��Cu2����Fe3��)���Ʊ��Ȼ���ͭ�Ĺ�������ͼ���£�
�������Ӻ�������ҺpH��CuCl��������ҺpH�Ĺ�ϵͼ��ͼ��
����֪����������Ũ��Ϊ1 mol��L��1ʱ��Fe(OH)3��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.4��3.0��Cu(OH)2��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.2��6.7����ش��������⣺
(1)���ʱ������Ӧ�����ӷ���ʽ��________������CuCl����ʱ�����pH��________���ҡ�
(2)���ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ____________________________��
(3)������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�����ո��������70�����2 h����ȴ�ܷ��װ��70����ո���ܷ��װ��Ŀ����____________________________________________��
(4)��Ʒ�˳�ʱ������Һ����Ҫ�ֳ���________���������Һ�л�ȡFeSO4��7H2O���壬����Ҫ֪������__________________��
(5)�������ۻ�����������Ҳ�ɵõ��Ȼ���ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��Ϊ���CuCl�IJ��ʣ����ڸ÷�Ӧ��ϵ�м���ϡ����Һ������pH��3.5����������Ŀ����__________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com