
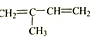 ��
��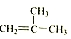
 ��
�� ��
�� D��
D��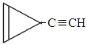 e��
e��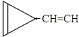 ��
�� ��
��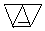 ��ֻ����һ���Ľṹ��ʽ����
��ֻ����һ���Ľṹ��ʽ���� ���� ��1��ͬϵ���ǽṹ���ƣ���������CH2ԭ���ŵ����ʻ���ͬϵ���ϩ����̼̼˫�����˵�ÿ��̼ԭ�����������������Ų�ͬʱ����ϩ������˳���칹��
��2���ܺ͵����ʵ��������������ӳɷ�Ӧ����2�ź�3��̼ԭ��֮����γ�һ��̼̼˫����
��3��ʵ�����õ�ʯ��ˮ��Ӧ��ȡ��Ȳ�������������Ȼ���Ϊ�����������£�����ȡ����Ӧ�������ȱ����Ȼ��⣻
��4������ͬϵ��ڽṹ��ֻ��һ������������Ϊ��������������ͨʽCnH2n-6�����ݵ�����Ϊ66�������ʽ��
��5��������������ֻ��C��H����Ԫ�أ���ij��B����14.3%����̼85.7%���ݴ�����л�������ʽΪCH2��80g��Br25%����ˮ��������ʵ���Ϊn=$\frac{80g��25%}{160g/mol}$=0.125mol�����л���ķ���ʽΪ��CH2��n����ˮ��������2.1g��Ϊ��ˮ���յĸ���������������1mol��CH2��n��1mol�������㣻
��6����C5H4�IJ����Ͷȣ�Ϊ4����̼ԭ�Ӿ�����ʱ��Ӧ��������ȲΪĸ��Ľṹ���������ĵ�Ч��ֻ��һ�֣�����л���Ľṹ�dz��Գƣ�
��� �⣺��1��ͬϵ���ǽṹ���ƣ���������CH2ԭ���ŵ����ʻ���ͬϵ����ڢľ�Ϊ�������ʽṹ���ƣ��ڷ�����������4��CH2ԭ���ţ��ʻ�Ϊͬϵ����л����д���̼̼˫������̼̼˫�����˵�ÿ��̼ԭ�����������������Ų�ͬʱ�����л������˳���칹����ֻ�Т�ClCH=CHCl����˳���칹������ľ�������˳���칹��
�ʴ�Ϊ���ڢޣ��ࣻ
��2����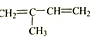 �͵����ʵ��������������ӳɷ�Ӧ������̼̼˫������Ȼ����2�ź�3��̼ԭ��֮����γ�һ��̼̼˫������2��̼ԭ������һ����������ΪCH3C��CH3��=CHCH3�����ò��������Ϊ��2-��-2-��ϩ��
�͵����ʵ��������������ӳɷ�Ӧ������̼̼˫������Ȼ����2�ź�3��̼ԭ��֮����γ�һ��̼̼˫������2��̼ԭ������һ����������ΪCH3C��CH3��=CHCH3�����ò��������Ϊ��2-��-2-��ϩ��
�ʴ�Ϊ��2-��-2-��ϩ��
��3��ʵ�����õ�ʯ��ˮ��Ӧ��ȡ��Ȳ������ʽΪ��CaC2+2H2O��Ca��OH��2+CH��CH����
�ʴ�Ϊ��CaC2+2H2O��Ca��OH��2+CH��CH����
�����������Ȼ���Ϊ�����������£������ϵ�һ����ԭ�ӱ�����ȡ���������ȱ����Ȼ��⣬��Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4������A�DZ���ͬϵ����ڽṹ��ֻ��һ���������Ҳ���Ϊ��������������ͨʽCnH2n-6�����ڵ�����Ϊ66�����У�6n+2n-6=66�����n=9����A�ķ���ʽΪC9H20������A�ı�����һ�����ֻ��һ�֣���A�Ľṹ�ܶԳƣ��ṹΪ ��
��
�ʴ�Ϊ�� ��
��
��5��������������ֻ��C��H����Ԫ�أ���ij��B����14.3%����̼85.7%���ʴ��л����е�C��Hԭ�Ӹ���֮��Ϊ��$\frac{14.3%}{1}$=1��2�����л���ķ���ʽΪ��CH2��n����ˮ��������2.1g��Ϊ��ˮ���յĸ��������������ʵ���n=$\frac{2.1g}{14ng/mol}$=$\frac{3}{20n}$mol����80g��Br5%����ˮ��������ʵ���Ϊn=$\frac{80g��5%}{160g/mol}$=0.025mol������1mol��CH2��n��1mol�壬���У�$\frac{3}{20n}$=0.025�����n=6�����ڷ�������������ȫ��Ч����B�Ľṹ��ʽΪ����CH3��2C=C��CH3��2��
�ʴ�Ϊ����CH3��2C=C��CH3��2��
��6����C5H4�IJ����Ͷȣ�Ϊ4����̼ԭ�Ӿ�����ʱ��Ӧ��������ȲΪĸ��Ľṹ������һ��̼̼�����IJ����Ͷ�Ϊ2����������������л����к�2��̼̼�������ʴ��л���ĽṹΪCH��C-C��C-CH3���������ĵ�Ч��ֻ��һ�֣�����л���Ľṹ�dz��Գƣ��ʿ���Ϊ ��
�� ��
��
�ʴ�Ϊ��CH��C-C��C-CH3�� ��
�� ��
��
���� �����ۺϿ�����ͬϵ�ͬ���칹����жϺ��л�����Ʊ��Լ�ͬ���칹�����д���ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ȡ��ҩƷ | B�� | 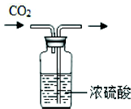 ���������̼ | C�� |  �ⶨij��Һ��pH | D�� |  �μ�Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 27 | B�� | 54.1 | C�� | 100 | D�� | 154 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȥNaCl��Һ������CaCl2����������K2CO3 | |
| B�� | ������ķ����Ӻ�ˮ�еõ���ˮ | |
| C�� | �þƾ����Դӵ�ˮ����ȡ�� | |
| D�� | ��Ũ�����ȥ�����л��е�ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼ԭ�Ӳ���һ��ֱ���� | B�� | �ȶ������Һ�� | ||
| C�� | 1mol������ȫȼ������������5mol | D�� | �������ܹ�����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��3a+b�� mol | B�� | ��3a+0.5b-3.5c�� mol | ||
| C�� | �� 3a+b+3.5c�� mol | D�� | ��3a+0.5b�� mol |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com