
ij��ѧ��ȤС���ͬѧ������ͼ��ʾʵ��װ�ý���ʵ��(ͼ��a��b��c��ʾֹˮ��)��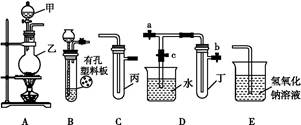
�밴Ҫ�����:
(1)����Bװ�ÿ���ȡ����������������(д�����ּ���)��
(2)A��C��E�������װ�ÿ�������ȡCl2��������ص�����ʵ�顣
�����ڱ��м�������ˮ,�����Ƶ���ˮ����������ˮ��Ϊ����,����ʵ���,ʵ�����������������:
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м��� NaHCO3��ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ�����н�ǿ������ |
(1)H2��CO2��
(2)��ʵ�����۲�����,��Ϊδ���и����Cl2�Ƿ���ʹƷ����ɫʵ��,��ʹƷ����ɫ�IJ�һ����������ˮ��Ӧ�IJ���,Ҳ������δ��Ӧ��������ʵ�����۲�����,��ȡ�������к���HCl����,HCl����ˮ������NaHCO3��ĩ��Ӧ�������ݡ����ڼס��ҡ����зֱ�װ��Ũ���ᡢMnO2��NaBr��Һ;��ƿ���л���ɫ��������,���Թ�����Һ����ɫ��Ϊ��ɫ;��֤��Cl2��������ǿ��Br2,��Br-�Ļ�ԭ��ǿ��Cl-
(3)��Cu+4HNO3(Ũ) Cu(NO3)2+2NO2��+2H2O��
Cu(NO3)2+2NO2��+2H2O��
��a��b��c��˫�ֽ���(����)�Թܶ�ʹ�Թ��������ݳ�,NO2��ˮ�Ӵ��������ձ��е�ˮ�������Թܶ���
��0.045 mol��L-1
����


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ����SO2��Ư�۾��ķ�Ӧ����ʵ��̽����
| ���� | ���� |
| ȡ����Ư�۾����壬����100mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ���������ɫ |
 | Һ���Ϸ����ְ����� �Ժ��ֻ��ǣ���Һ��Ϊ����ɫ�� �Ժ���������ɫ����������ɫ��ȥ |
| ����� | 1 | 2 | 3 |
| KI��Һ���/mL | 19.98 | 20.02 | 20.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧϰ��ȤС��̽����������ȡʵ��:
(1)��ͬѧ��������ʵ�鷽���Ʊ�����,���к������� (�����,��ͬ)��
| A�����Ȼ�粒�����ȷֽ� |
| B����Ũ��ˮ�����������ƹ����� |
| C�����������ƹ������Ũ��ˮ�� |
| D�����Ȼ��ϡ��Һ�����������ƹ����� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��������ϵ�֪��Ư�������ᷴӦ�����Ƶ���������ѧ����ʽΪ��Ca(ClO)2+CaCl2+2H2SO4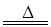 2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣
2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣
�Իش�(1)��ʵ����A���ֵ�װ���ǣߣߣߣ�(��дװ�õ����)��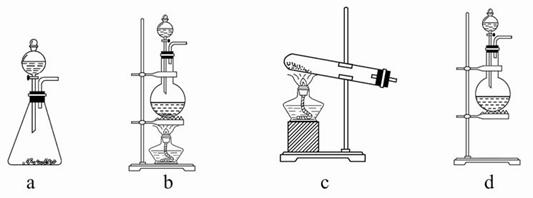
(2)�������һ��ʵ�飬֤��ϴ��ƿC�е�Na2SO3�Ѿ�������(����ʵ�鲽��)���ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(3)��D�з�Ӧ�����Һ����Ư���ԣ���д��Dװ���з�����Ӧ�����ӷ���ʽ
�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
(4)��ʵ��������Ե�ȱ�ݣ���������Ľ��ķ����ߣߣߣߣߣߣߣߣߣߣߡ�
(5)��С���ֽ���������ʵ�飺��ȡƯ��2.0 g����ĥ���ܽ⣬���Ƴ�250 mL��Һ��ȡ25 mL���뵽��ƿ�У��ټ��������KI��Һ������H2SO4��Һ�����á�����ȫ��Ӧ����0.1 mol/L��Na2S2O3��Һ������Һ�ζ���Ӧ���ɵĵ⣬��֪��ӦʽΪ�� 2Na2S2O3+I2=Na2S4O6 + 2NaI ��Ӧ���ʱ������ȥNa2S2O3
20.0 mL�����Ư����Ca(ClO)2����������Ϊ���ߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ�ϳ�����������ʢװ��Ũ���ᡣΪ�о����ʲ�������Ũ����ķ�Ӧ��ijѧϰС�����������̽�����
��̽��һ��
��1������ȥ�������������������̼�ظ֣�������Ũ�����У�10���Ӻ���������ͭ��Һ�У�Ƭ�̺�ȡ���۲죬�������������Ա仯����ԭ����__________________��
��2����ȡ����6.0 g����15.0 mLŨ�����У����ȣ���ַ�Ӧ���ռ�������Y��
��ͬѧȡ336 mL����״��������Yͨ��������ˮ�У�������Ӧ��SO2+Br2+2H2O=2HBr+H2SO4
Ȼ���������BaCl2��Һ�����ʵ�������ø������2.33 g���ɴ���֪����Y��SO2���������Ϊ______��
��̽������
��������ʵ����SO2��������Ľ������ͬѧ��Ϊ����Y�л����ܺ���H2��Q���塣Ϊ�����������̽��ʵ��װ�ã�ͼ�мг�����ʡ�ԣ���
��3��װ��B���Լ���������_________________________________________��
��4����Ϊ����Y�л�����Q��������______________________________�����û�ѧ����ʽ��ʾ����
��5��Ϊȷ��Q�Ĵ��ڣ�����װ��������M��______��ѡ����ţ���
a.A֮ǰ b.A��B�� c.B��C�� d.C��D��
��6���������Y�к���H2��Ԥ��ʵ������Ӧ��____________________________��
��7����Ҫ�ⶨ���������Y��H2�ĺ�������״����Լ��28 mL H2���������ò���H2����ķ����⣬�ɷ�ѡ�����������ķ����������жϲ�˵������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС���������Ѿ�ѧ���Ļ�ѧ֪ʶ������װ����ȡ�����������ƣ�����һ��̽���������ƵĻ�ѧ���ʡ�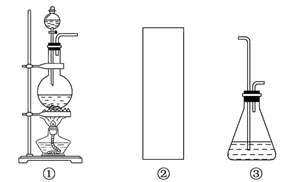
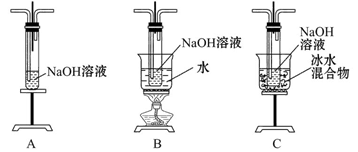
������ϣ���ӦCl2+2NaOH=NaClO+NaCl+H2O���ڷ��ȷ�Ӧ���¶��Ը�ʱ�㷢������Ӧ��3Cl2+6NaOH=NaClO3+5NaCl+3H2O��
(1)��ȡ����ʱ������ƿ�м���һ�����Ķ������̣�ͨ��________(��д��������)����ƿ�м���������Ũ���ᡣ����װ��A��B��C��ѡ��һ�����ʵ�װ�÷��ڢڴ�________��
(2)����ѧ����Ϊ����װ�ô���ȱ�㣬����ָ����ǰ��������֮��Ӧ������Dͼ��ʾ��װ�ã�����ΪD����ʢҺ����________����������____________��
(3)�û�ѧ��ȤС��Բ�Ʒ�����ʽ���������̽����
��һ��������pH=10�����������ӷ���ʽ����ԭ��________��
�ڶ������û�ѧ��ȤС���ͬѧ��ѡ����ɫʯ����Һ�Դ������Ƶ����ʽ���ʵ����顣��������������ʵ�鱨�棺
| ʵ����� | Ԥ������ | ���� |
| | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣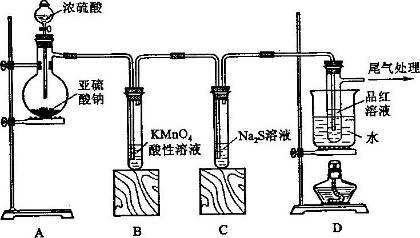
��ش��������⣺
��1��װ��A��ʢ���������Ƶ�����������_________�����з�����Ӧ�Ļ�ѧ����ʽ_______________________��
��2��ʵ������У�װ��B��C�з���������ֱ���____________��___________��װ��B�з�����Ӧ�����ӷ���ʽΪ_________________________________��
��3��װ��D��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������________________________��
��4��β���ɲ���___________________��Һ���ա�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ����С��������ͼװ��̽��SO2�����ʡ�
��ش��������⣺
��1��װ�ü���A������������_____________����ƿ�ڷ�����Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��2��װ�����г��ֵ�������_______________________________������֤��SO2����______������ţ���װ�ñ��з�����Ӧ�Ļ�ѧ����ʽΪ______________________������֤��SO2����______������ţ���
A�������� B����ԭ�� C��Ư����
��3���ռ�SO2�������ѡ���װ��Ϊ______������ţ���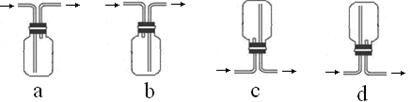
�ӻ����ĽǶȿ��ǣ��ռ�װ�õij�������Ҫ����һ��ʢ��_________���ѧʽ����Һ��ϴ��ƿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧУ��ѧѧϰС��Ϊ̽���������������ʣ�����ͼ��ʾװ�ý���ʵ�飬��ش���������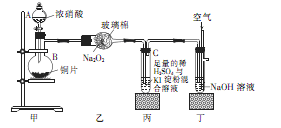
��1��װ�ü���ʢ��Ũ���������A�������� ������B�з�����Ӧ�Ļ�ѧ����ʽΪ
��2�������������еĿհ�
��3��ȡ��װ�ñ��е��Թ�C�������еμ�������Na2SO3��Һ����Һ��ɫ��ȥ���ù����з�����Ӧ�����ӷ���ʽΪ ����Ӧ�����Һ����Ҫ����SO32����SO42����I���������ӣ�����д����SO32����SO42����I����ʵ�鱨�档
��ѡ�Լ���2mol��L��1HCl��1mol��L��1H2SO4��1mol��L��1BaCl2��1mol��L��1Ba(NO3)2��CCl4�����Ʊ�����ˮ�����Ʊ�����ˮ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com