
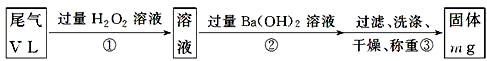



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ��ʵ | ���� |
| A | �����۳�����Ũ���� | Ũ�����ǿ������ʹ���ۻ� |
| B | �ñ���NH4Cl��Һ����������̨�����ɷ��� | NH4Cl�ֽ����ȣ��ҷֽ�����ܸ��������е����� |
| C | ��Ԫ�ص���ɫ��Ӧ�ʻ�ɫ | Na2O2��һ�ֵ���ɫ�Ĺ��� |
| D | SO2��ʹ��ˮ��ɫ | SO2���л�ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͨ���������Ϊdz��ɫ���壬������Ϊ����ɫ������ |
| B��������ˮ����������ˮ |
| C����������������Ӧʱ����ԭΪ��2�ۣ����ȱ���ԭΪ��1�� |
| D����Cu��Fe��Ӧʱ���������������ɵͼ�̬�������������ɸ�̬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ļ�ԭ�� | B�����Ư���� |
| C����������Ļ�ԭ�� | D�����������Ư���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ʱ��������������Ӧ���ų����� |
| B������ʱ������ͭ������Ӧ |
| C����Ԫ�صĻ��ϼ۶���+6�� |
| D��������Ϊ����ĸ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2 | B��NH3 | C��HCl | D��SO3�����壩 |
�鿴�𰸺ͽ���>>
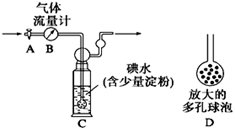
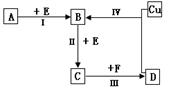
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�� HClO | B�� H2O2 | C�� O3 | D�� SO2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com