
����Ŀ��A(C3H6)�ǻ����л�����ԭ�ϡ���A�Ʊ��ۺ���C��![]() �ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
�ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��
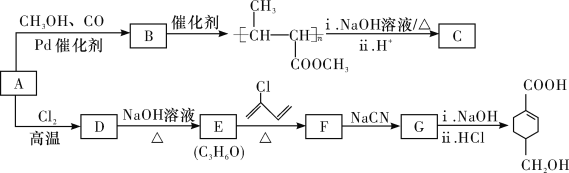
��֪��
��![]() +
+![]()
![]()
![]()
��R��C��N![]() R��COOH
R��COOH
�ش��������⣺
(1)D��������___________��B���еĺ��������ŵ�������__________��
(2)C�Ľṹ��ʽΪ_____________��D��E�ķ�Ӧ����Ϊ ____________��
(3)E��F�Ļ�ѧ����ʽΪ___________��
(4)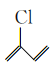 �������_____��ԭ�ӹ�ƽ�棬
�������_____��ԭ�ӹ�ƽ�棬![]() �������۷�Ӧ�����л���Ľṹ��ʽΪ__________��
�������۷�Ӧ�����л���Ľṹ��ʽΪ__________��
(5)B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ�Ĺ���_______��(�����������칹)�����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6:1:1����__________(д�ṹ��ʽ)��
(6)��������Ϣ������ϩ��HBrΪ��ʼԭ���Ʊ����ᣬ��ƺϳ�·��(�����Լ���ѡ)________��
���𰸡�3-�ȱ�ϩ ���� 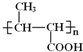 ȡ����Ӧ��ˮ�ⷴӦ
ȡ����Ӧ��ˮ�ⷴӦ 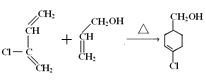 10
10 ![]() �� 8
�� 8 ![]() C H2=C H2
C H2=C H2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH
CH3CH2COOH
��������
B�����Ӿ۷�Ӧ���ɾ۶�ϩ���������B�ṹ��ʽΪCH3CH=CHCOOCH3��AΪC3H6��A���������ӳɷ�Ӧ����B����A�ṹ��ʽΪCH2=CHCH3���۶�ϩ���������ˮ�ⷴӦȻ���ữ�õ��ۺ���C��C�ṹ��ʽΪ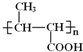 ��A������Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ��������Ϣ�ļӳɷ�Ӧ�����E����ʽ֪��E�ṹ��ʽΪCH2=CHCH2OH��D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ
��A������Ӧ����D��D����ˮ�ⷴӦ����E��E�ܷ��������Ϣ�ļӳɷ�Ӧ�����E����ʽ֪��E�ṹ��ʽΪCH2=CHCH2OH��D�ṹ��ʽΪCH2=CHCH2Cl��E��2-��-1��3-����ϩ�����ӳɷ�Ӧ����F��F�ṹ��ʽΪ![]() ��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ�
��F����ȡ����Ӧ����G��G������Ϣ�з�Ӧ�õ� ����G�ṹ��ʽΪ
����G�ṹ��ʽΪ![]() ��
��
��6��CH2=CH2��HBr�����ӳɷ�Ӧ����CH3CH2Br��CH3CH2Br��NaCN����ȡ����Ӧ����CH3CH2CN��CH3CH2CN�ڼ��������·���ˮ�ⷴӦȻ���ữ�õ�CH3CH2COOH���ݴ˷������
1. �ɷ�����֪D�ṹ��ʽΪCH2=CHCH2Cl����������3-�ȱ�ϩ��B�ṹ��ʽΪCH3CH=CHCOOCH3��B�к���������������������
�ʴ�Ϊ��3-�ȱ�ϩ��������
2. �ɷ�����֪C�ṹ��ʽΪ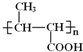 ��D����ȡ����Ӧ��ˮ�ⷴӦ����E��
��D����ȡ����Ӧ��ˮ�ⷴӦ����E��
�ʴ�Ϊ�� 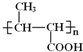 ��ȡ����Ӧ��ˮ�ⷴӦ��
��ȡ����Ӧ��ˮ�ⷴӦ��
3.E�ṹ��ʽΪCH2=CHCH2OH��F�ṹ��ʽΪ![]() ��E�����ӳɷ�Ӧ����F���÷�Ӧ����ʽΪ
��E�����ӳɷ�Ӧ����F���÷�Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
4. �÷����к���10��ԭ�ӣ�������ϩ�ṹ�ص�֪���÷���������ԭ�ӹ�ƽ�棻���л�������۷�Ӧ����ṹ��ʽΪ![]() ��
��
�ʴ�Ϊ��10��![]() ��
��
5. B�ṹ��ʽΪCH3CH=CHCOOCH3��B��ͬ���칹���У���B������ͬ�Ĺ��������ܷ���������Ӧ��˵������̼̼˫����������ȩ����Ϊ������������������ͬ���칹����HCOOCH=CHCH2CH3 HCOOCH2CH=CHCH3 HCOOCH2CH2CH=CH2 HCOOC(C H3)=CHCH3 HCOOCH=C(CH3)2 HCOOC(CH3)CH=CH2 HCOOCHC(CH3)=CH2 HCOOCH(CH2CH3)=CH2�����Է�����������8�֣����к˴Ź�������Ϊ3��壬�ҷ����֮��Ϊ6��1��1����![]() ��
��
�ʴ�Ϊ��8��![]() ��
��
6. C H2=CH2��HBr�����ӳɷ�Ӧ����CH3CH2Br��CH3CH2Br��NaCN����ȡ����Ӧ����CH3CH2CN��CH3CH2CN�ڼ��������·���ˮ�ⷴӦȻ���ữ�õ�CH3CH2COOH��������ϳ�·��Ϊ
C H2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH��
CH3CH2COOH��
�ʴ�Ϊ��C H2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2CN
CH3CH2CN![]() CH3CH2COOH��
CH3CH2COOH��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
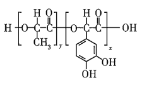
����Ŀ���߾���![]() ���ṹ��ʽ��ͼ��ʾ����һ����Դ������ѧ��е�����ճ�ϼ�����ԭ��ȡ����ֲ����ݱ������й��ڸ߾���
���ṹ��ʽ��ͼ��ʾ����һ����Դ������ѧ��е�����ճ�ϼ�����ԭ��ȡ����ֲ����ݱ������й��ڸ߾���![]() ��˵��������ǣ� ��
��˵��������ǣ� ��
A.�߾���![]() �ĵ���֮һΪ
�ĵ���֮һΪ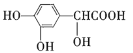
B.�߾���![]() ��һ���������ܷ���ˮ�ⷴӦ
��һ���������ܷ���ˮ�ⷴӦ
C.�����ۺϷ�Ӧʱ������![]() ��ͬʱ������
��ͬʱ������![]() ����
����
D.![]() ������
������![]() �����ӳɷ�Ӧ
�����ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȼ��0.1 molij�л����0.2 mol CO2��0.3 mol H2O���ɴ˵ó��Ľ��۲���ȷ����(����)
A. ���л�����ӵĽṹ��ʽΪCH3��CH3
B. ���л�����̼����Ԫ��ԭ����Ŀ֮��Ϊ1��3
C. ���л�������в����ܺ���![]() ˫��
˫��
D. ���л�������п��ܺ�����ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����CF3COOHˮ��Һ�н�����ֱ��ת��ΪCF3COOCH3��ˮ������CH3OH���ķ�Ӧ������ͼ������˵����ȷ����
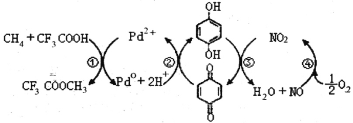
A.��������Ԫ�ص�һ�����ܣ�C<N<O<F
B.��Ӧ����������Ϊ�Ա�����
C.������Ӧ���ܷ�ӦʽΪ��CH4+CF3COOH+![]() O2
O2 CF3COOCH3+H2O
CF3COOCH3+H2O
D. ��������Ũ��ˮ��Ӧ���ҷ�Ӧ������ͬ
��������Ũ��ˮ��Ӧ���ҷ�Ӧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Cinanserin����������70������ڿ��������֢��ҩ��Թ�״����3CLˮ��ø�����������ã���ͼ����ϳ�·��ͼ��

��֪������Ϣ��
��AΪ��������������H2������ܶ�Ϊ39
��2RCOOH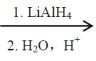 RCH2OH
RCH2OH
��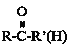 +HCN
+HCN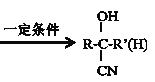
�ش��������⣺
��1��A������__________��B�Ľṹ��ʽ___________��������к��������ŵ�����__________��
��2������ٵķ�Ӧ����ʽ________________���䷴Ӧ����Ϊ_________��
��3�� �й�ƽ���ԭ�����________����
�й�ƽ���ԭ�����________����
��4��M��C��ͬ���칹�壬��������������M��_______�֡�
�����ڷ����廯������ܷ���������Ӧ��
��5��������ɣ�д����![]() �Ʊ�
�Ʊ�![]() ������ͼ��_______��
������ͼ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��CH3CH2CH2CH2OH![]() CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������������
���� | �е�/�� | �ܶ� / gcm-3 | ˮ���ܽ��� |
|
������ | 117.2 | 0.8109 | �� | |
����ȩ | 75.7 | 0.8017 | �� |
����˵���У�����ȷ����
A.Ϊ��ֹ�����һ��������Ӧ���ữ��Na2Cr2O7��Һ��μ�����������
B.���¶ȼ�1ʾ��Ϊ90~95�����¶ȼ�2ʾ����76������ʱ���ռ�����
C.��Ӧ������������ﵹ���Һ©���У���ȥˮ�㣬������ȩ�ӷ�Һ©���Ͽڵ���
D.���õĴ�����ȩ�м������������ƣ����������Ƿ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)��һ�ֽⶾҩ�����ڷ�����顢����Ǧ����������ж����ٴ�����������ݡ���Ƥ�������Ȳ�֢.��������������Ի���Ի������ȶ�����������Һ�зֽ����S��SO2
ʵ��I��Na2S2O3���Ʊ�����ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��

(1)����a��������_______������b��������_______��b��������������Ϊ70%80%��H2SO4��Һ��Na2SO3���巴Ӧ�Ʊ�SO2��Ӧ�Ļ�ѧ����ʽΪ_______��c���Լ�Ϊ_______
(2)ʵ����Ҫ����SO2���������ʣ����Բ�ȡ�Ĵ�ʩ��_______ (д��һ��)
(3)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ����_______
ʵ���̽��Na2S2O3����������ӵ�������ԭ��Ӧ��
���ϣ�Fe3++3S2O32-Fe(S2O3)33-(�Ϻ�ɫ)
װ�� | �Լ�X | ʵ������ |
| Fe2(SO4)3��Һ | ��Ϻ���Һ�ȱ���Ϻ�ɫ��30s����Ϊ��ɫ |
(4)��������ʵ���������ж�����Fe3+��S2O32-��ԭΪFe2+��ͨ��_______(��������Լ�������)����һ��֤ʵ������Fe2+���ӻ�ѧ��Ӧ���ʺ�ƽ��ĽǶȽ���ʵ��������_______
ʵ��궨Na2S2O3��Һ��Ũ��
(5)��ȡһ�������IJ�Ʒ���Ƴ������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽȷ��ȡ������K2Cr2O7(Ħ������Ϊ294gmol-1)0.5880g��ƽ���ֳ�3�ݣ��ֱ����3����ƿ�У���ˮ�����Һ�������������KI���ữ���������з�Ӧ��6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������ӦI2+2S2O32- = 2I- + S4O62-���������� Na2S2O3��Һ��ƽ�����Ϊ25.00 mL�������궨�������������Һ��Ũ��Ϊ_______molL-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����CH3��3 NH+��![]() ���γ�����Һ�塣����Һ����������������ɣ��۵����100�棬��ӷ���һ����л��ܼ�___________��������������С������������___________������ţ���
���γ�����Һ�塣����Һ����������������ɣ��۵����100�棬��ӷ���һ����л��ܼ�___________��������������С������������___________������ţ���
a.��ȼ�� b.����ɫ���ܼ� c.���ϲ��� d.���Ȳ���
��2��Ϊ���о��������Ŀռ���ˮ�Ľ���¶ȣ���ѧ�ҶԲ�ֱͬ��̼������ˮ�Ľ���¶Ƚ��з�������ͼ�����ֲ�ֱͬ��̼�����еı����ṹ������¶ȣ������Ĵ�Сȡ����̼���ܵ�ֱ����ˮ��̼�����н���Ĺ�����_________________��
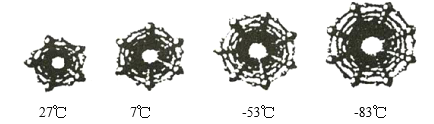
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ij����ֻ��C��H��O����Ԫ�أ������ģ����ͼ��ʾ�������й���12��ԭ�ӡ�
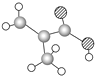
�����ʵĽṹ��ʽΪ_______________�������������������ŵ�����Ϊ______________________��
��2�����и������ʣ� �� O2��O3 �� H2��D2��T2 �� 12C��14C �� CH3CH2CH2CH3 �� (CH3)2CH2CH3 ������Ͷ��� ��CH3CH2CH2CH��C2H5��CH3��CH3CH2CH2CH��CH3��C2H5 ��Ϊͬϵ�����__________�� ��Ϊͬ���칹�����________����Ϊͬλ�ص���________�� ��Ϊͬ�����������_________����ͬһ���ʵ���__________��
��3����̪�dz��õ����ָʾ������ṹ��ʽ��ͼ��ʾ��
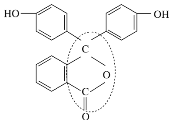
�ٷ�̪�ķ���ʽΪ___________________________________��
�ڴӽṹ�Ϸ�����̪�ɿ���_____________________��
A��ϩ�������������� B�����㻯����
C���������� D����������
E���������� F����������
�۷�̪�ṹ��ʽ�л����ߵĵط����Ѽ���_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com