
��ͼ��ʾһЩ�������ʼ���ת����ϵ������C��E���ǽ������ʣ�������HΪ��ɫҺ�壬C��B��ϡ��Һ����Ӧ��B��K��ͬһ�������ʣ�����ɫ����������F��Lʱ�����Ϊ��ɫ��������M������Һ�벡����Һ���ȣ�����ϲ����Ƿ��������Իش�
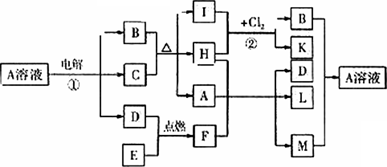
(1)F�ĵ���ʽΪ________����Ӧ�ٵ�������ӦʽΪ________��
(2)��Ӧ�ڵĻ�ѧ����ʽΪ________��
(3)B��Һ��M��Ӧ�����ӷ���ʽΪ________��
(4)I��F��һ����ͬ����;��________��
(5)����M������Һ�������ǹ��������ڣ�������Ӧ�Ļ�ѧ����ʽΪ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�����������ѧУ2008������꼶�ڶ���������⻯ѧ�Ծ� ���ͣ�022
��ͼ��ʾһЩ�������ʼ���ת����ϵ������C��E���ǽ������ʣ�������HΪ��ɫҺ�壬C��B��ϡ��Һ����Ӧ��B��K��ͬһ�������ʣ�����ɫ����������F��Lʱ�����Ϊ��ɫ��������M������Һ�벡����Һ���ȣ�����ϲ����Ƿ��������Իش�

(1)F�ĵ���ʽΪ________����Ӧ�ٵ�������ӦʽΪ________��
(2)��Ӧ�ڵĻ�ѧ����ʽΪ________��
(3)B��Һ��M��Ӧ�����ӷ���ʽΪ________��
(4)I��F��һ����ͬ����;��________��
(5)����M������Һ�������ǹ��������ڣ�������Ӧ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
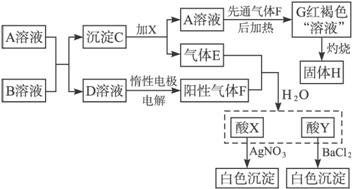
��1����д���������ʵĻ�ѧʽ��C___________��H___________��
��2��E��F������������ˮ����X+Y�����ӷ���ʽΪ________________________�����D��Һ�����ӷ���ʽΪ_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ������У������ѧ���� ���ͣ������
��8�֣���ͼΪһЩ�������ʼ���ת����ϵ������FΪ����������������ijЩ���������ﷴӦ���ǹ�ҵұ�������ķ���֮һ�� AΪ��ɫ���壬BΪ����ɫ���壬CΪ��ɫҺ�壬E��JΪ��ɫ���嵥�ʡ�

�Իش��������⣺
��1��д��B�Ļ�ѧʽ�� ��д��A�ĵ���ʽ ��
��2��д��I��K�����ӷ���ʽ ��
��3��д��F��Fe3O4�ڸ����·�Ӧ�Ļ�ѧ����ʽ ��
��4����֪3g J��ȫȼ�շų�a kJ����������д����ʾJ��ȼ���ȵ��Ȼ�ѧ����ʽ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ��ƶ���

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com