
 2MgO£»¢ع3Mg+N2
2MgO£»¢ع3Mg+N2 Mg3N2£»¢غ2Mg+CO2
Mg3N2£»¢غ2Mg+CO2 2MgO+C
2MgO+C MgO+H2،ü£»¢فMg3N2+6H2O
MgO+H2،ü£»¢فMg3N2+6H2O 3Mg(OH)2+2NH3،ü
3Mg(OH)2+2NH3،ü 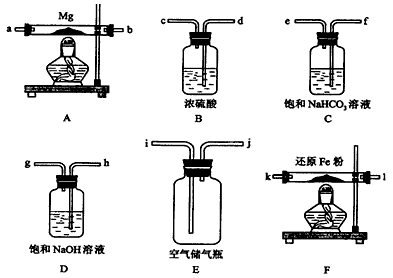



| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛°²»صت،ثقضفتذءéèµضذر§2009½ى»¯ر§µعب´خشآ؟¼تشجâ جâذح£؛058
دضؤâشعتµرéتزہïہûسأ؟صئّ؛حأ¾·غخھشءدضئب،ةظء؟µھ»¯أ¾(Mg3N2)£®زرضھتµرéضذ؟ةؤـ»ل·¢ةْدآءذ·´س¦£؛
¢ظ2Mg£«O2![]() 2MgO£»
2MgO£»
¢ع3Mg£«N2![]() Mg3N2£»
Mg3N2£»
¢غ2Mg£«CO2![]() 2MgO£«C
2MgO£«C
¢ـMg£«H2O![]() MgO£«H2،ü
MgO£«H2،ü
¢فMg3N2£«6H2O![]() 3Mg(OH)2£«2NH3،ü
3Mg(OH)2£«2NH3،ü
؟ة¹©ر،شٌµؤ×°ضأ؛حز©ئ·بçدآح¼ثùت¾(أ¾·غ،¢»¹شجْ·غ¾ùزر¸ةشװضأؤعثù·¢ةْµؤ·´س¦تاحêب«µؤ£¬صûج××°ضأµؤؤ©¶ثسë¸ةشï¹ـدàء¬)£®

»ط´ًدآءذختج⣻
(1)شعةè¼ئتµرé·½°¸ت±£¬³×°ضأA،¢Eح⣬»¹س¦ر،شٌµؤ×°ضأ(جî×ضؤ¸´ْ؛إ)¼°ئنؤ؟
________،،________________________________
________،،________________________________
________،،________________________________
________،،________________________________
(ثµأ÷£؛؟ةزش²»جîآْ£¬؟صبô²»¹»£¬ز²؟ةزشجي¼س£®)
(2)ح¨ئّ؛َ£¬بç¹ûح¬ت±µمب¼A،¢F×°ضأµؤ¾ئ¾«µئ£¬¶شتµرé½ل¹ûسذ؛خس°دى£؟________£¬شزٍتا________£»
(3)اëةè¼ئز»¸ِ¼ٍµ¥µؤذ،تµر飬؟ةزشرéض¤²ْخïتاµھ»¯أ¾£؛________
²é؟´´ً°¸؛ح½âخِ>>
°ظ¶بضآذإ - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com