
| |||||||||||||||||||
(1) |
����(4��)ֻҪд������4�����ӷ���ʽ�е������������ɣ� ����HCO��3��OH�� ����Ca2+��CO32�� ����Ca2+��2HCO3����2OH�� ����Mg2+��2OH�� |
(2) |
��(1��),Fe(OH)3(1��)Fe3+��3H2O��Fe(OH)3(����)��3H+(2��) |
(3) |
(2��)��ȥ������,������Һ��� |
(4) |
(1��)ɱ������(��������),(1��)�� |


 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| PH | 6.5��8.5 |
| Ca2+��Mg2+��Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/mL |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��17�֣��ҹ��涨����ˮ�����������������Ҫ��
| PH | 6��5~8��5 |
| Ca2����Mg2����Ũ�� | ��0.0045mol/L |
| ϸ������ | ��100��/mL |
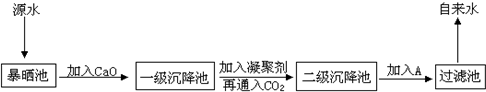
ij�ۺ�ʵ���С�鵽����ˮ���ιۣ��˽Դˮ����������ˮ�Ĺ�������ʾ��ͼ���£�
��1��Դˮ�к���Ca2����Mg2����HCO3-��Cl-�ȣ�����CaO�������ɸ���ѧ��Ӧ����д����������һ����Ӧ�����ӷ���ʽ��_____________________________________
��2���������������Գ�ȥ���е���������������ù�����____________________������ţ�
�� ֻ���������̣���ѧ����
�� ֻ�л�ѧ���̣�����������
�� �����������̣����л�ѧ����
��3��FeSO4��7H2O�dz��õ���������������������ɺ��ɫ��״�����������ֳ�����___________���ѧʽ��
��4��ͨ�������̼��Ŀ����__________��___________��
��5������A��������_________����A����ѡ�����������е�__________������ţ�
��ClO2 ��SO2 ��Һ�� ��Ca(ClO2)2 ��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��17�֣��ҹ��涨����ˮ�����������������Ҫ��
|
PH |
6��5~8��5 |
|
Ca2����Mg2����Ũ�� |
��0.0045mol/L |
|
ϸ������ |
��100��/mL |
ij�ۺ�ʵ���С�鵽����ˮ���ιۣ��˽Դˮ����������ˮ�Ĺ�������ʾ��ͼ���£�
 [��Դ:ZXXK]
[��Դ:ZXXK]
��1��Դˮ�к���Ca2����Mg2����HCO3-��Cl-�ȣ�����CaO�������ɸ���ѧ��Ӧ����д����������һ����Ӧ�����ӷ���ʽ��_____________________________________
��2���������������Գ�ȥ���е���������������ù�����____________________������ţ�
�� ֻ���������̣���ѧ����
�� ֻ�л�ѧ���̣�����������
�� �����������̣����л�ѧ����
��3��FeSO4��7H2O�dz��õ���������������������ɺ��ɫ��״�����������ֳ�����___________���ѧʽ��
��4��ͨ�������̼��Ŀ����__________��___________��
��5������A��������_________����A����ѡ�����������е�__________������ţ�
��ClO2 ��SO2 ��Һ�� ��Ca(ClO2)2 ��Ũ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com