
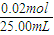 =0.80mol/L���ڢ��У�����0.005mol��˵���ڶ���Ӧ������ȥ0.005mol�������һ��Ӧ�ľ���0.015mol����ô̼���Ƶ�������0.015mol��Ũ��Ϊ��
=0.80mol/L���ڢ��У�����0.005mol��˵���ڶ���Ӧ������ȥ0.005mol�������һ��Ӧ�ľ���0.015mol����ô̼���Ƶ�������0.015mol��Ũ��Ϊ��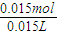 =1.00mol/L���ʴ�Ϊ��0.80��1.0��
=1.00mol/L���ʴ�Ϊ��0.80��1.0��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ��Ŀ�� | ���鷽�� |
| �����Ȼ������Ƿ���� | D D |
| ��ȥʳ��������ϸɳ | C C |
| ��ȥ̼���ƹ���������̼������ | A A |
| ��ȥþ���л��е��������� | B B |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�Ƹ���ѧ����ʯ����2007�����������ѧ�Ծ� ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش�(1)�����������ֲ�ͬ�������̼�ʵ�����ݿ��жϼ���Һ��___________������Һ��___________��
(2)�����ӷ���ʽ��ʾ���β����õ���ͬ���������ԭ��
��_______________________________________________________��
��_______________________________________________________��
(3)����Һ�����ʵ���Ũ��Ϊ___________mol��L-1��(CO2��ˮ��Һ�е������ܽ���Բ���)
(4)��nmL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ��������������ΪVmL(��״��)����V��ȡֵ��ΧΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش�(1)�����������ֲ�ͬ�������̼�ʵ�����ݿ��жϼ���Һ��___________������Һ��___________��
(2)�����ӷ���ʽ��ʾ���β����õ���ͬ���������ԭ��
��_______________________________________________��
��_______________________________________________��
(3)����Һ�����ʵ���Ũ��Ϊ___________mol��L-1������Һ�����ʵ���Ũ��Ϊ_______mol��L-1(CO2��ˮ��Һ�е������ܽ���Բ���)��
(4)��n mL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ������������壻��ΪV mL(��״��)����y��ȡֵ��ΧΪ___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com