
 ��֪ˮ�ĵ���ƽ��������ͼʾ���Իش��������⣺
��֪ˮ�ĵ���ƽ��������ͼʾ���Իش��������⣺���� ��1��Kwֻ���¶��йأ��¶���ͬ���ӻ�������ͬ���¶�Խ�ߣ����ӻ�����Խ��
��2����A�㵽D��c��H+�����c��OH-����С����Kw���䣻
��3��E��Ӧ���¶��£�Kw=10-14����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7��������Һ�е�n��H+�����ڼ���Һ�е�n��OH-�����ݴ˼��㣻
��4�����¶���ˮ�����ӻ�ΪKw=1��10-12����ͼ��ϣ�0.05mol/L��ϡ������Һ��������Ũ��Ϊ1mol/L�������û��Һ��pH=2���������������c��H+��=$\frac{n��{H}^{+}��-n��O{H}^{-}��}{V}$���㣬��ʽ����pH���ɼ�������ȣ�
��5������Һ��������Ǹ�����Һ��H+Ũ����OH-Ũ�ȵ���Դ�С�жϵģ�ֻҪ��Һ��c��H+��=c��OH-������Һ�ͳ����ԣ�CH3COOH��������ʣ�����̶Ȳ���NaOH��ǿ����ʣ���ȫ���룬��Ӧ���ɵ���������ǿ��������ˮ��ʼ��ԣ�����Һ�����ԣ����ټӼ������Һ������pH=7c��H+��=c��OH-����ϵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�����н��
�ڵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-���������غ�c��Na+��=2[c��CH3COO-��+c��CH3COOH��]��
��6��c��Cu2+��=$\frac{{K}_{sp}}{c��O{H}^{-}��^{2}}$��
��� �⣺��1��ˮ�����ӻ�����ֻ���¶��йأ��¶�Խ�ߣ����ӻ�����Խ��ͬһ��������ͬ�¶ȣ�����ͼ֪���¶ȸߵ͵�˳����B��C��A=D=E���������ӻ�������С˳����B��C��A=D=E���ʴ�Ϊ��B��C��A=D=E��
��2����A��ʱ��c��H+��=c��OH-������Һ�����ԣ�����D��c��H+�����c��OH-����С����Һ�����ԣ�����A�㵽D�㣬��Һ�����Ա�Ϊ���ԣ���Kw���䣮
a�������¶ȣ�Kw���a����
b�������������ᣬ����Һ�����ԣ���b��ȷ��
c�������Ȼ�泥�ˮ�������ԣ���Kw���䣬��c��ȷ��
�ʴ�Ϊ��bc��
��3��E��Ӧ���¶��£�Kw=10-14����pH=9��NaOH��Һ��pH=4��H2SO4��Һ��ϣ������û����Һ��pH=7��������Һ�е�n��H+�����ڼ���Һ�е�n��OH-�������У�
10-5mol/L��V��=10-4mol/L��V������ã�$\frac{V���}{V���ᣩ}$=10��1��
�ʴ�Ϊ��10��1��
��4�����¶���ˮ�����ӻ�ΪKw=1��10-12����pH=11�Ŀ�����������������Ũ��Ϊ��c��OH-��=$\frac{1��1{0}^{-12}}{1��1{0}^{-11}}$mol/L=0.1mol/L��0.05mol/L��ϡ������Һ��������Ũ��Ϊ1mol/L��pH=2����Һ��������Ũ��Ϊ0.01mol/L������Һ�����㣺0.1mol/L��V2-0.1mol/L��V1=0.01mol/L����V1+V2����
�����ɵã�V1��V2=9��11��
�ʴ�Ϊ��9��11��
��5����CH3COOH��������ʣ�����̶Ȳ���NaOH��ǿ����ʣ���ȫ���룬��Ӧ���ɵ���������ǿ�������Σ�ˮ��ʼ��ԣ�����Һ������pH=7�����ټӼ���Գ����£���V mL��0.1000mol•L-1����������Һ��μ��뵽20.00mL��0.1000mol•L-1������Һ�У���ַ�Ӧ��V��20.00mL��Һ������pH=7��c��H+��=c��OH-�������ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-����c��H+��=c��OH-������Һ�е�����Ϊ��������Һ��ˮ�ĵ��������ģ�����c��Na+��=c��CH3COO-����c��H+��=c��OH-����
�ʴ�Ϊ������c��Na+��=c��CH3COO-����c��H+��=c��OH-����
�ڸ��ݵ���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-���������غ�c��Na+��=2[c��CH3COO-��+c��CH3COOH��]���õ�c��H+��+c��CH3COO-��+2c��CH3COOH��=c��OH-������c��OH-��-c��H+��-c��CH3COOH��=c��CH3COO-��+c��CH3COOH������Ӧ����Һ�������Ϊ60mL����c��CH3COO-��+c��CH3COOH���T$\frac{0.1000mol/L��20mL}{60mL}$=0.033mol/L��
�ʴ�Ϊ��0.033��
c��Cu2+��=$\frac{{K}_{sp}}{c��O{H}^{-}��^{2}}$=$\frac{2.2��1{0}^{-20}}{��\frac{1{0}^{-14}}{1{0}^{-8}}��^{2}}$mol/L=2.2��10-8mol/L��
�ʴ�Ϊ��2.2��10-8��
���� ���⿼������ϵĶ����жϡ���ҺpH���йؼ��㣬���ؿ���ѧ�������жϼ�������������ȷ�����ʱ������ʵ����Ĺ�ϵ����ҺpH��c��H+������OH-���Ĺ�ϵ�ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 0.1mol/LNaHCO3��Һ��0.1mol/LNaOH��Һ�������ϣ�������Һ�У�c��Na+����c��HCO3-����c��OH-����c��CO32-�� | |
| B�� | 20ml0.1mol/LCH3COONa��Һ��10ml0.1mol/LHCl��Һ��Ϻ�����ԣ�������Һ�У�c��CH3COO-����c��Cl-����c��CH3COOH����c��H+�� | |
| C�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��NH4+����c��Cl-����c��OH-����c��H+�� | |
| D�� | 0.1mol/LCH3COOH��Һ��0.1mol/LNaOH��Һ�������ϣ�������Һ�У�c��OH-����c��H+��+c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�22.4 L H2O�к�����ԭ����ĿΪ2NA | |
| B�� | 17 g NH3����������Ϊ10NA | |
| C�� | 0.1mol Cu��NO3��2�к��е�������ĿΪ0.2NA | |
| D�� | 28g N2 ���Ϊ22.4L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ý�̿��ԭSiO2��ȡ�ֹ� | |
| B�� | ������۵��Ӳ�ȴ���˿����������뵼����� | |
| C�� | ʯӢֻ�������������ά | |
| D�� | ���������ɳ���ʢ�Ÿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ñ���ȡ��ˮ�е��� | B�� | ��������ˮ | ||
| C�� | ʯ�͵����� | D�� | ʳ������ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
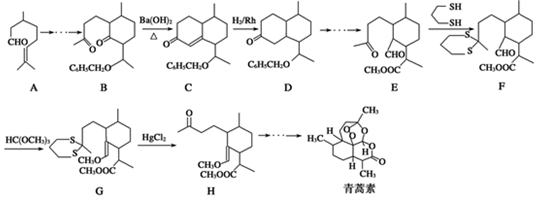
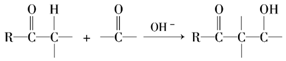 ��
��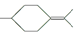 ���������մ��Ľṹ��ʽΪ
���������մ��Ľṹ��ʽΪ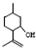 ��
�� ���ĺϳ�·������ͼ�����Լ���ѡ����
���ĺϳ�·������ͼ�����Լ���ѡ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
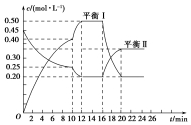 ��һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA��g��+2B��s��?yC��g����H��0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
��һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA��g��+2B��s��?yC��g����H��0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ҵ�ϴӷ�Ǧ���ص�Ǧ�����Ǧ�Ĺ����У�����̼������Һ��Ǧ�ࣨ��Ҫ�ɷ�ΪPbSO4��������Ӧ��PbSO4��s��+CO32-��aq��?PbCO3��s��+SO42-��aq����ij��������PbSO4Ϊԭ��ģ��ù��̣�̽��������Ӧ��ʵ���������������ijɷ֣�
��ҵ�ϴӷ�Ǧ���ص�Ǧ�����Ǧ�Ĺ����У�����̼������Һ��Ǧ�ࣨ��Ҫ�ɷ�ΪPbSO4��������Ӧ��PbSO4��s��+CO32-��aq��?PbCO3��s��+SO42-��aq����ij��������PbSO4Ϊԭ��ģ��ù��̣�̽��������Ӧ��ʵ���������������ijɷ֣�| ʵ�鲽�裨��Ҫ��д������������̣� | Ԥ�ڵ�ʵ������ͽ��� |
| ȡһ������Ʒ��ָ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com