
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪:X+Y![]() Z+W
Z+W
(1)Y�ĵ���ʽ��_________________________��
(2)Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ��_________________________________��
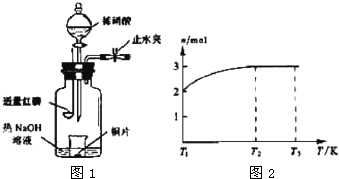
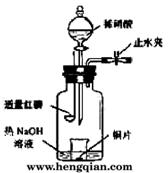 (3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
a.����ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
�ٲ���c��ȱ�ٵ�һ����Ҫ������_______________________________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ____________________________________________________________________��
�۲���c����ϡ������ձ��е�������______________________________________
______________________________________________________________________��
��Ӧ�����ӷ���ʽ��____________________________________________________��
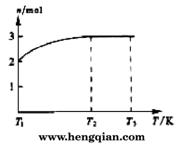 (4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2,����ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
(4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2,����ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
���¶���T1-T2֮�䣬��Ӧ�Ļ�ѧ����ʽ��_________________________��
���¶���T2-T3֮�䣬�����ƽ����Է��������ǣ�����1λС����______________��
(1) ![]() (2)2NH3(l) ? NH
(2)2NH3(l) ? NH![]() NH
NH![]()
(3) �ٴ�ֹˮ�У�ͨ���������� ��P2O5+6OH-=2PO![]() +3H2O
+3H2O
��CuƬ���ܽ⣬����ɫ���ݲ�������Һ����ɫ��Ϊ��ɫ
3Cu+8H++2NO![]() =3Cu2++2NO
=3Cu2++2NO![]() +4H2O
+4H2O
(4) ��2NO2 ?2NO+O2 ��30.7
X��Y��Z��W������ͬ�������ķ��ӻ����ӣ������Ԫ�ص�������С��10��X��5��ԭ�Ӻˣ�ΪCH4��NH4����ͨ��״���£�WΪ��ɫҺ�壬ΪH2O������![]() ����֪XΪNH4����YΪOH����ZΪNH3����Y�ĵ���ʽΪ��
����֪XΪNH4����YΪOH����ZΪNH3����Y�ĵ���ʽΪ��![]() ��Һ�������ˮ���ƣ������ʽΪ��2NH3(l)=NH4����NH2������ͼʾװ���Ʊ�NO����֤�仹ԭ�ԣ�������������û����֤���裬�������Ʊ���NO��ͨ������������ͨ������仯ȷ���仹ԭ�ԣ�����c���������ӣ���ֹˮ�У�ͨ���������������׳��ȼ������P2O5�������������Ʒ�ӦΪ�� P2O5��6OH��=2PO43����3H2O������c��������������Ϊ��CuƬ���ܽ⣬����ɫ���ݲ�������Һ����ɫ��Ϊ��ɫ����ӦΪ��3Cu��8H����2NO3��=3Cu2����2NO��4H2O��1molN2O4�����ܱ������У����¹����У���ת��Ϊ����ɫ��˵��N2O4=2NO2����ʱװ����Ϊ2molNO2������ͼ���б�ʾ�����壬������ѹʱ����������ʵ������࣬��Ϊ��ɫ����T2�¶Ⱥ����ʵ���ԼΪ3mol�����䷴ӦʽΪ��2NO2=O2��2NO������Է�������Ϊ��30.7��
��Һ�������ˮ���ƣ������ʽΪ��2NH3(l)=NH4����NH2������ͼʾװ���Ʊ�NO����֤�仹ԭ�ԣ�������������û����֤���裬�������Ʊ���NO��ͨ������������ͨ������仯ȷ���仹ԭ�ԣ�����c���������ӣ���ֹˮ�У�ͨ���������������׳��ȼ������P2O5�������������Ʒ�ӦΪ�� P2O5��6OH��=2PO43����3H2O������c��������������Ϊ��CuƬ���ܽ⣬����ɫ���ݲ�������Һ����ɫ��Ϊ��ɫ����ӦΪ��3Cu��8H����2NO3��=3Cu2����2NO��4H2O��1molN2O4�����ܱ������У����¹����У���ת��Ϊ����ɫ��˵��N2O4=2NO2����ʱװ����Ϊ2molNO2������ͼ���б�ʾ�����壬������ѹʱ����������ʵ������࣬��Ϊ��ɫ����T2�¶Ⱥ����ʵ���ԼΪ3mol�����䷴ӦʽΪ��2NO2=O2��2NO������Է�������Ϊ��30.7��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y
X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮��֪��X+Y
| ||
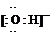
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2008?������X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮
��2008?������X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˣ�ͨ��״���£�WΪ��ɫҺ�壮| �� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008��߿��������ۻ�ѧ ���ͣ�ʵ����
(17��)X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪:X+Y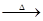 Z+W
Z+W
(1)Y�ĵ���ʽ��_________________________��
(2)Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ��_________________________________��
(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������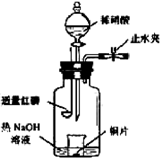
a.����ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b.�ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c.�����׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
�ٲ���c��ȱ�ٵ�һ����Ҫ������_______________________________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ____________________________________________________________________��
�۲���c����ϡ������ձ��е�������______________________________________
______________________________________________________________________��
��Ӧ�����ӷ���ʽ��____________________________________________________��
(4)һ���¶��£���1 mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2,����ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��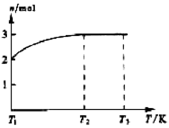
���¶���T1-T2֮�䣬��Ӧ�Ļ�ѧ����ʽ��_________________________��
���¶���T2-T3֮�䣬�����ƽ����Է��������ǣ�����1λС����______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʦ���и������Ĵ��¿���ѧ�Ծ� ���ͣ������
��12�֣�X��Y��Z��WΪ������ͬ�������ķ��ӻ����ӣ�����ԭ������С��10��Ԫ����ɣ�X��5��ԭ�Ӻˡ�ͨ��״���£�WΪ��ɫҺ�塣
��֪��X+Y Z+W
Z+W
��1��Z�Ŀռ乹��Ϊ ��
��2��Һ̬Z��W�ĵ������ƣ����ɵ������������ͬ���������ӣ�Һ̬Z�ĵ��뷽��ʽ�� ��
��3��1mol��̬Z��O2��Ӧ����Һ̬W��һ��������Ԫ����ɵ��������ʣ��ų�������ΪQkJ��д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4��һ���¶��£���1mol N2O4�����ܱ������У�����ѹǿ���䣬�����¶���T1�Ĺ����У���������ɫ��Ϊ����ɫ���¶���T1�������ߵ�T2�Ĺ����У�������Ϊ��ɫ��������T2������ѹǿ��������Ϊ����ɫ����������ʵ���n���¶�T�仯�Ĺ�ϵ��ͼ��ʾ��
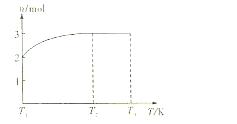
���¶���T1��T2֮�䣬��Ӧ�Ļ�ѧ����ʽ�ǣ� ��
���¶���T2��T3֮�䣬�����ƽ����Է��������ǣ�����1λС���� ��
������ʵ�����õ�ƽ��������ͨ��������ˮ�У���ʹ���屻��ȫ����������Ӧͬʱͨ���״���µĿ��� L������������Ϊ��N2��O2�������4��1��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com