
½«32.64gĶÓė140mLŅ»¶ØÅØ¶ČµÄĻõĖį·“Ó¦£¬ĶĶźČ«Čܽā²śÉśµÄNOŗĶNO2»ģŗĻĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ11.2L”£Ēė»Ų“š£ŗ
£Ø1£©NOµÄĢå»żĪŖ L£¬NO2µÄĢå»żĪŖ L”£
£Ø2£©“ż²śÉśµÄĘųĢåČ«²æŹĶ·Åŗó£¬ĻņČÜŅŗ¼ÓČėVmL a mol/LµÄNaOHČÜŅŗ£¬Ē”ŗĆŹ¹ČÜŅŗÖŠµÄCu2£«Č«²æ×Ŗ»Æ³É³Įµķ£¬ŌņŌĻõĖįČÜŅŗµÄÅضČĪŖ mol/L”£
£Ø3£©ÓūŹ¹ĶÓėĻõĖį·“Ӧɜ³ÉµÄĘųĢåŌŚNaOHČÜŅŗÖŠČ«²æ×Ŗ»ÆĪŖNaNO3£¬ÖĮÉŁŠčŅŖ30%µÄĖ«ŃõĖ®
gӣ
£Ø1£©5.8 5.4 £Ø2£© (av.10-3+0.5)/0.14 £Ø3£©57.67
½āĪöŹŌĢā·ÖĪö£ŗĶÓėŅ»¶ØÅØ¶ČµÄĻõĖį·“Ó¦ĖłÉę¼°µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ
Cu+4HNO3(ÅØ)=Cu(NO3)2+2H2O+NO2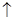 £»3Cu+8HNO3(Ļ”)=3Cu(NO3)2+4H2O+2NO
£»3Cu+8HNO3(Ļ”)=3Cu(NO3)2+4H2O+2NO
£Ø1£©ÉčNOµÄĢå»żĪŖ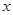 L£¬ŌņNO2µÄĢå»żĪŖ
L£¬ŌņNO2µÄĢå»żĪŖ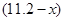 L£¬ÓÉÉĻŹö·½³ĢŹ½æÉÖŖ¢ŁÓėĻ”ĻõĖį·“Ó¦µÄĶµÄÖŹĮæĪŖ
L£¬ÓÉÉĻŹö·½³ĢŹ½æÉÖŖ¢ŁÓėĻ”ĻõĖį·“Ó¦µÄĶµÄÖŹĮæĪŖ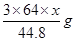 £»¢ŚÓėÅØĻõĖį·“Ó¦µÄĶµÄÖŹĮæĪŖ
£»¢ŚÓėÅØĻõĖį·“Ó¦µÄĶµÄÖŹĮæĪŖ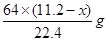 ”£Ņņ“ĖæɵĆČēĻĀµČŹ½£ŗ
”£Ņņ“ĖæɵĆČēĻĀµČŹ½£ŗ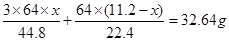 £¬½āµĆ
£¬½āµĆ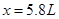 ”£Ņņ“ĖNOµÄĢå»żĪŖ5.8L£»NO2µÄĢå»żĪŖ5.4L”£
”£Ņņ“ĖNOµÄĢå»żĪŖ5.8L£»NO2µÄĢå»żĪŖ5.4L”£
£Ø2£©ČÜŅŗÖŠĶĄė×ÓµÄĪļÖŹµÄĮæ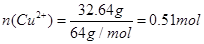 £¬Ņņ“ĖŌĻõĖįµÄĪļÖŹµÄĮæÅضČ
£¬Ņņ“ĖŌĻõĖįµÄĪļÖŹµÄĮæÅضČ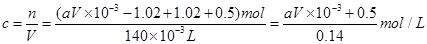
£Ø3£©·¢ÉśµÄ·“Ó¦ĪŖ2NO2+H2O2=2HNO3”¢2NO+3H2O2=2HNO3+2H2O”£ÉčÓėNO2·“Ó¦µÄH2O2µÄÖŹĮæĪŖ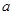 £¬ÓėNO·“Ó¦µÄH2O2µÄÖŹĮæĪŖ
£¬ÓėNO·“Ó¦µÄH2O2µÄÖŹĮæĪŖ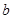 £¬ŌņæɵĆČēĻĀ¹ŲĻµŹ½£ŗ
£¬ŌņæɵĆČēĻĀ¹ŲĻµŹ½£ŗ £¬½āµĆ
£¬½āµĆ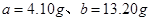 £¬Ņņ“ĖŠčŅŖ30%µÄĖ«ŃõĖ®µÄÖŹĮæĪŖ
£¬Ņņ“ĖŠčŅŖ30%µÄĖ«ŃõĖ®µÄÖŹĮæĪŖ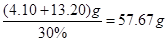 ”£
ӣ
æ¼µć£ŗĶÓėĻõĖįµÄ·“Ó¦
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖĶÓėĻõĖį·“Ó¦ÖŠĖłÉę¼°µ½µÄ¼ĘĖć£¬ŹōÓŚ»ł“”Ģā”£½āĢāµÄ¹Ų¼üŌŚÓŚÕżČ·Š“³ö»Æѧ·½³ĢŹ½£¬ŌŚ¼ĘĖćµÄ¹ż³Ģ֊ӦעŅā¹ŲĻµŹ½·Ø”¢ŹŲŗć·ØµÄÓ¦ÓĆ”£


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| aV”Į10-3+0.5 |
| 0.14 |
| aV”Į10-3+0.5 |
| 0.14 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
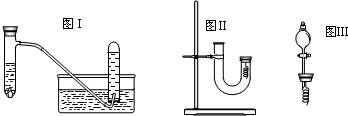
| ŹµŃé²½Öč | ĪŹĢā |
| 1“ÓUŠĶ¹Ü×ó¶Ė¼ÓČėĻ”ĻõĖįÖ±ÖĮ³äĀśUŠĶ¹ÜÓŅ¶Ė | ”Ī/ |
| 2ÓĆø½ÓŠĶĖæµÄ½ŗČūČū×”UŠĶ¹ÜÓŅ¶Ė£¬¹Ū²ģĻÖĻó | ĻÖĻóŹĒ ÓŠĪŽÉ«ĘųĢå²śÉś£¬ÓŅ±ßČÜŅŗÖš½„±ä³ÉĀĢÉ« ÓŠĪŽÉ«ĘųĢå²śÉś£¬ÓŅ±ßČÜŅŗÖš½„±ä³ÉĀĢÉ« |
| 3“ż·“Ó¦Ķ£Ö¹ŗó“ņæŖ½ŗČū£¬¹Ū²ģŹµŃéĻÖĻó | ĻÖĻóŹĒ ĪŽÉ«ĘųĢåÓėæÕĘų½Ó“„ŗóĮ¢¼“±ä³Éŗģ×ŲÉ« ĪŽÉ«ĘųĢåÓėæÕĘų½Ó“„ŗóĮ¢¼“±ä³Éŗģ×ŲÉ« |
| 10-3a©qV +0.5 |
| 0.14 |
| 10-3a©qV +0.5 |
| 0.14 |
| 1 |
| 2 |
|
|
| 1 |
| 2 |
|
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģŗžÄĻŹ”ŌĄŃōŹŠŅ»ÖŠøßČżµŚŅ»“ĪÖŹĮæ¼ģ²ā»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗ¼ĘĖćĢā
ĻõĖįŹĒ³£¼ūµÄČż“óĒæĖįÖ®Ņ»£¬ŌŚ»Æѧъ¾æŗĶ»Æ¹¤Éś²śÖŠÓŠ×Źć·ŗÓ¦ÓĆ£¬³£ÓĆÓŚÖʱøĻõĖįŃĪ”¢Č¾ĮĻ”¢·ŹĮĻ”¢Ņ½Ņ©ÖŠ¼äĢ唢ĮŅŠŌÕØŅ©µČ”£ĻõĖįŃĪ¶ąÓĆÓŚŃ껚”¢ŹŌ¼Į”¢Ķ¼Ļń“¦ĄķŠŠŅµ”£
£Ø1£©Ä³½šŹōMµÄĻõĖįŃĪŹÜČČŹ±°“ĻĀŹ½·Ö½ā£ŗ2MNO3 2M+2NO2”ü+O2”ü£¬¼ÓČČ3.40gMNO3£¬Éś³ÉNO2ŗĶO2ÕŪĖć³É±ź×¼×“æöŹ±µÄ×ÜĢå»żĪŖ672mL”£ÓÉ“ĖæÉŅŌ¼ĘĖć³öMµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ_____________”£
2M+2NO2”ü+O2”ü£¬¼ÓČČ3.40gMNO3£¬Éś³ÉNO2ŗĶO2ÕŪĖć³É±ź×¼×“æöŹ±µÄ×ÜĢå»żĪŖ672mL”£ÓÉ“ĖæÉŅŌ¼ĘĖć³öMµÄĻą¶ŌŌ×ÓÖŹĮæĪŖ_____________”£
£Ø2£©½«32.64gĶÓė140mL Ņ»¶ØÅØ¶ČµÄĻõĖį·“Ó¦£¬ĶĶźČ«Čܽā²śÉśµÄNOŗĶNO2»ģŗĻĘųĢåÕŪĖć³É±ź×¼×“æöĻĀµÄĢå»żĪŖ11.2L”£ĘäÖŠNOµÄĢå»żĪŖ_____________”£
£Ø3£©ĻÖÓŠCu”¢Cu2OŗĶCuO×é³ÉµÄ»ģŗĻĪļ£¬Ä³ŃŠ¾æŠŌѧĻ°Š”×éĪŖĮĖĢ½¾æĘä×é³ÉĒéæö£¬¼ÓČė100mL0.6mol/LHNO3ČÜŅŗĒ”ŗĆŹ¹»ģŗĻĪļĶźČ«Čܽā£¬Ķ¬Ź±ŹÕ¼Æµ½224mLNOĘųĢå£Ø±ź×¼×“æö£©”£Ōņ²śĪļÖŠĻõĖįĶµÄĪļÖŹµÄĮæĪŖ ”£ČēŌ»ģŗĻĪļÖŠÓŠ0.0lmolCu£¬ŌņĘäÖŠCu2OÓėCuOµÄÖŹĮæ±ČĪŖ_____________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com