


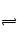 HS2O3�D��OH�D,�������������һ�����൱���������е��������ˮ�������������ˮ��Һ�����ԡ�
HS2O3�D��OH�D,�������������һ�����൱���������е��������ˮ�������������ˮ��Һ�����ԡ�

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2C��g������2�����C��Ũ��Ϊ0.6 mol?L-1��������
2C��g������2�����C��Ũ��Ϊ0.6 mol?L-1��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
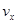 ����x����ij��Ӧ��������֮��Ĺ�ϵ����ȷ���ǣ� ��
����x����ij��Ӧ��������֮��Ĺ�ϵ����ȷ���ǣ� ��A�� �� �� | B�� �� �� | C�� �� �� | D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��b ="1/2" a | B��b ="2/3" a | C��b ="3/2" a | D��b ="2" a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��VA��3.0mol��L��1��min��1 | B��VB��0.28mol��L��1��S��1 |
| C��VC��4.8mol��L��1��min��1 | D��VD��1.0mol��L��1��S��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Al��������ȼ������A12O3����Al�۸�ΪAlƬ |
| B��Fe��ϡ���ᷴӦ��ȡH2ʱ������������Ũ����ͬ��ϡ���� |
| C��Zn��ϡ���ᷴӦʱ���ʵ������Һ���¶� |
| D��Na��ˮ��Ӧʱ����ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 4NO��6H2O��5 L���ܱ������н��У�����Ӻ�NO�����ʵ���������0.3 mol����˷�Ӧ��ƽ������
4NO��6H2O��5 L���ܱ������н��У�����Ӻ�NO�����ʵ���������0.3 mol����˷�Ӧ��ƽ������ (��ʾ��Ӧ����������ʻ����������������)Ϊ�� ��
(��ʾ��Ӧ����������ʻ����������������)Ϊ�� �� A�� �� 0.01 mol / (L��s) �� 0.01 mol / (L��s) | B�� �� 0.008 mol / (L��s) �� 0.008 mol / (L��s) |
C�� �� 0.003 mol / (L��s) �� 0.003 mol / (L��s) | D�� �� 0.004 mol / (L��s) �� 0.004 mol / (L��s) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
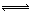 4NO��g����6H2O��g������������ȷ����
4NO��g����6H2O��g������������ȷ����| A���ﵽƽ��ʱ��4v��(O2)=5v��(NO) |
| B������λʱ��������x mol NO��ͬʱ������x mol NH3,��Ӧ�ﵽƽ��״̬�� |
C���ﵽƽ��ʱ�������������ݻ���������Ӧ���ʼ�С���淴Ӧ������ �� �� |
| D����ѧ��Ӧ�����ʹ�ϵ�ǣ�2v��(NH3)=3v��(H2O) |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com