
| 0.92g |
| 46g/mol |
| 0.24g |
| 0.002mol |
| 6.0g |
| 120g/mol |
| 3.36L |
| 22.4L/mol |
| 1.8g |
| 18g/mol |
 £®
£® £®
£®


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
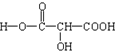
£Ø6·Ö£©Äɶ¹ŹĒŅ»ÖÖ¼õ·ŹŹ³Ę·£¬“ÓĘäÖŠ·ÖĄė³öŅ»ÖÖÓÉC”¢H”¢OČżÖÖŌŖĖŲ×é³ÉµÄÓŠ»śĪļA£¬ĪŖČ·¶ØĘä½į¹¹ĻÖ½ųŠŠČēĻĀø÷ŹµŃé£ŗ
¢Ł6.0gAŌŚŅ»¶ØĢõ¼žĻĀĶźČ«·Ö½ā£¬Éś³É3.36L£Ø±ź×¼×“æöĻĀ£©Ņ»Ńõ»ÆĢ¼ŗĶ1.8gĖ®£»
¢ŚÖŠŗĶ0.24gĪļÖŹAĻūŗÄ0.2mol/LµÄNaOHČÜŅŗ20.00mL£»
¢Ū0.01molĪļÖŹAĶźČ«×Ŗ»ÆĪŖõ„£¬ŠčŅŖŅŅ“¼0.92g£¬0.01molAÄÜÓė×ćĮæÄĘ·“Ó¦·Å³ö0.336L£Ø±ź×¼×“æöĻĀ£©ĒāĘų”£
Ķعż¼ĘĖćČ·¶Ø£ŗ¢ÅAµÄĦ¶ūÖŹĮæŹĒ AµÄ»ÆѧŹ½ĪŖ
¢ĘAµÄ½į¹¹¼ņŹ½ŹĒ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com