

| Ũ���� |
| �� |
| Ũ���� |
| �� |
| һ������ |
| һ������ |


| Ũ���� |
| �� |
| һ������ |
| Ũ���� |
| �� |
| һ������ |
 ������������-OH������Cԭ����û��Hԭ�ӣ�����Cu��Ag�������������²��ܱ������������ʴ�Ϊ��
������������-OH������Cԭ����û��Hԭ�ӣ�����Cu��Ag�������������²��ܱ������������ʴ�Ϊ�� ����
����

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
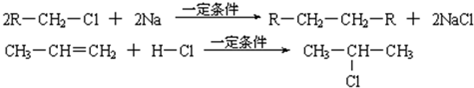
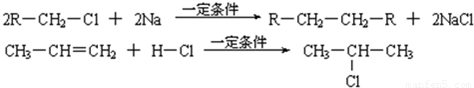
��֪����1������ȩ��ֱ��������̼ԭ���ϵ����Ϊ��Hԭ�ӣ���ϡ����Һ�Ĵ������£�һ��ȩ�����ϵĦ�Hԭ�����ӵ���һ��ȩ���ӵ���ԭ���ϣ����ಿ�����ӵ��ʻ�̼ԭ���������ǻ�ȩ���磺
R��CH2��CHO+R1��CH2��CHO![]()

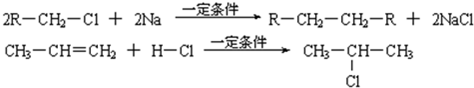
��2��2R��CH2��Cl+2Na![]() R��CH2��CH2��R+2NaCl
R��CH2��CH2��R+2NaCl
��3��CH3��CH=CH2+H��Cl![]()
![]()
�ϳ�·�����£�

��1���ڷ�Ӧ�ڡ����У����ڼӳɷ�Ӧ����____________��������ȥ��Ӧ����____________��
��2��д�����з�Ӧ����ʽ
E![]() F��_________________________________________________________��
F��_________________________________________________________��
H![]() G��_________________________________________________________��
G��_________________________________________________________��
��3��HҲ����NaOH��Һ��Ӧ���䷴Ӧ����ʽΪ__________________�����ɵ��л����ܷ���Cu��Ag�������������±���������__________����ܡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���ٷ���ȩ��ֱ��������̼ԭ���ϵ���ԭ�ӱ���Ϊ��-Hԭ�ӣ���ϡ���ϡ����Һ�Ĵ�������(�������ϡ����Һ����)��һ����ȩ�ϵĦ�-Hԭ�Ӽӳɵ���һ����ȩ����ԭ���ϣ����ಿ�ּӳɵ��ʻ�̼ԭ�������ɦ�-�ǻ�ȩ�������Ӧ�ͽ�����ȩ���ϣ���
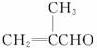
��2R��CH2Cl+2Na![]() R��CH2��CH2��R+2NaCl
R��CH2��CH2��R+2NaCl
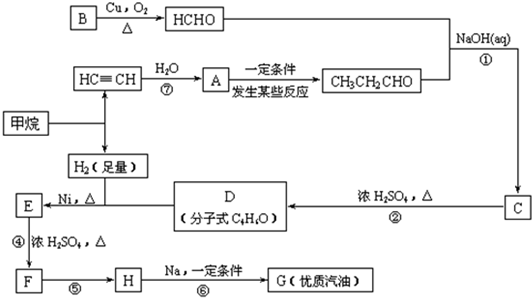
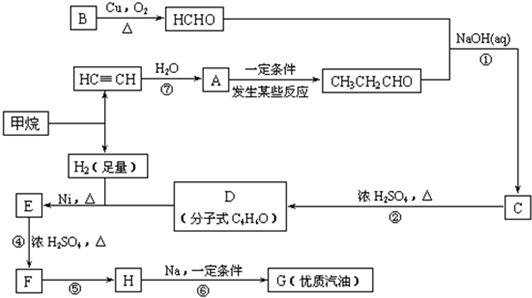
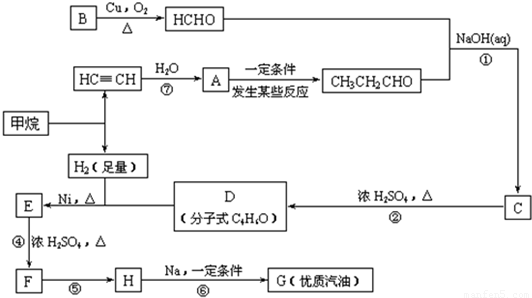
�ϳ�·����ͼ1-6-6��ʾ
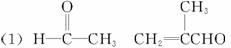
ͼ1-6-6
(1)д���������ʵĽṹ��ʽ��A___________��D___________��
(2)д���ϳ�·�����йصķ�Ӧ���ͣ���___________����___________��
(3)��д�йػ�ѧ��Ӧ����ʽ��
E��F��______________________��
CH3C(CH3)2Cl��G��___________��
(4)д��C��ͬ���칹��Ľṹ��ʽ(�������������������)��___________��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
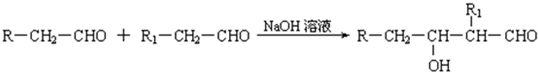
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����������������ѧ�߿���ѧ��ģ�Ծ��������棩 ���ͣ������
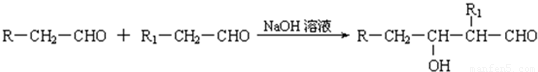
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com