
 ȷ��ȡ25.00mLijδ֪Ũ�ȵ�NaOH��Һ��һ�ྻ��ƿ�У�Ȼ����0.20mol/L��������Һ�ζ���ָʾ��Ϊ���ȣ����ζ�������£�
ȷ��ȡ25.00mLijδ֪Ũ�ȵ�NaOH��Һ��һ�ྻ��ƿ�У�Ȼ����0.20mol/L��������Һ�ζ���ָʾ��Ϊ���ȣ����ζ�������£�| HCl��Һ��ʼ���� | HCl��Һ�յ���� | |
| ��һ�� | 2.15mL | |
| �ڶ��� | 3.10mL | 21.85mL |
| ������ | 4.20mL | 22.95mL |
���� ��1�����ݵζ��ܵĽṹ�;�ȷ�ȶ�����
��2��������ʽ�ζ��������ݵķ���������ʽ�ζ���������б��Ѹ�ٴ�������������Һ�����������ų���
��3�����ж����ݵĺ����ԣ�Ȼ��NaOH��Һ��ƽ�������Ȼ�����HCl��NaOH����������ʵ���Ũ�ȣ�
��4��ָʾ��Ϊ���ȣ���ɫ��ΧΪ3.1-4.4��
��5������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
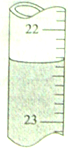
��� �⣺��1���ζ��ܵ���̶������棬�ζ����е�Һ�����Ϊ22.40mL��
�ʴ�Ϊ��22.40��
��2����ȥ��ʽ�ζ��������ݵľ�������ǣ�����ʽ�ζ���������б��Ѹ�ٴ�������������Һ�����������ų���
�ʴ�Ϊ������ʽ�ζ���������б��Ѹ�ٴ�������������Һ�����������ų���
��3��3�����ĵ�HCl��Һ�����Ϊ20.25mL��18.75mL��18.75mL����һ����ȥ��HCl��Һ��ƽ�����Ϊ18.75mL
HCl��NaOH
1 1
0.20mol/L��18.75mL c��NaOH����25.00mL
��ã�c��NaOH��=0.15mol/L
�ʴ�Ϊ��0.15��
��4��ָʾ��Ϊ���ȣ���ɫ��ΧΪ3.1-4.4���յ�ʱpHԼΪ4.4����Һ�ɻ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ�Ϊԭ������ɫ��
�ʴ�Ϊ���ƣ��ȣ�
��5��A���ζ���������ˮϴ��δ�ñ���Һ��ϴ��ֱ��װ�����Һ������Һ��ϡ�ͣ����V������ƫ��c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ��A��ȷ��
B���ζ��ܶ���ʱ���ζ�ǰ���ӵζ����ӣ����V������ƫС��c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫС����B����
C���ζ�ǰ�ζ��ܼ�������ݣ��κ�������ʧ�����V������ƫ��c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c�����⣩ƫ��C��ȷ��
D���ζ����յ㸽��ʱ������������ˮ��ϴ��ƿ�ڱ���մ������Һ������Һ���ʵ������䣬��V��������Ӱ�죬c�����⣩���䣬��D����
��ѡAC��
���� ���⿼������к͵ζ�ʵ�飬�ѶȲ���ע�����ʵ���ԭ�������衢�����Լ�ע���������ʵ�����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 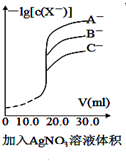 ����������Һ�ζ���Ũ�ȵ�A-��B-��C-�Ļ����Һ����������Ag+��Ӧ���ɳ���������ͼ��ȷ�����ȳ�������C- | |
| B�� | 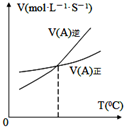 ͼ��ʾ��Ӧ��ij��Ӧ��������淴Ӧ�������¶ȱ仯�������ͼ��֪�÷�Ӧ������Ӧ�����ȷ�Ӧ | |
| C�� | 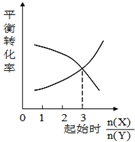 һ�������£�X��Y��Ӧ����Z����ͼ1�Ƴ��÷�Ӧ�ķ���ʽ�ɱ�ʾΪ��X+3Y?Z | |
| D�� | 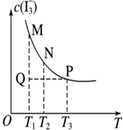 ͼ��ʾ��Һ�з�Ӧ��I2+I-?I3- ƽ��c��I3-�����¶ȱ仯����Ӧ�ٶ�V������M��V���棩N |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | W | ||
| X | Y | �� | Z |
| A�� | ���Ӱ뾶��X��Y��Z��W | |
| B�� | ��̬�⻯����ȶ���W��Z | |
| C�� | Ԫ��X��Z�γɵĻ�������ֻ�������Ӽ� | |
| D�� | ����������Ӧˮ����ļ���X��Y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu+4HNO3�TCu��NO3��2+2NO2��+2H2O | B�� | 2NaOH+CuSO4�TNa2SO4+Cu��OH��2�� | ||
| C�� | 2CO+O2�T2CO2 | D�� | 2Al+2NaOH+2H2O�T2NaAlO2+3H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �ڢ����ӷ���ʽΪNa++NH3+H2O+CO2��NaHCO3��+NH4+ | |
| B�� | �ڢõ��ľ�����Na2CO3•10H2O | |
| C�� | A������CO2��B������NH3 | |
| D�� | �ڢ��������Ĺ�����Ҫ���ܽ⡢�������ᾧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
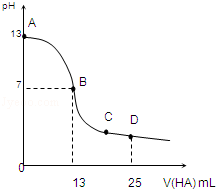 �����£���25mL 0.1mol/L MOH��Һ����μ���0.2mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺
�����£���25mL 0.1mol/L MOH��Һ����μ���0.2mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | δ֪Ũ�������õζ�����ȡ���õζ���������ˮϴ�Ӻ�δ�ô���Һ��ϴ | |
| B�� | װ����Һ����ƿ��ϴ�Ӻ�δ�ɾ�ʢ�����Һ | |
| C�� | �ζ��������ø��ӷ��۲��ʽ�ζ��̶ܿȣ����������ȷ | |
| D�� | �ζ��������ּ�ʽ�ζ��ܼ��촦����һ�μ�Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com