
����Ŀ��ijУ��ѧС���ͬѧ��չ��һϵ�еĻ�ѧʵ����

�������ʵ�鲢�����������:
(1)��ͬѧ��ͼ1��ʾװ��,����п�����ᷴӦ����:��2 gп��������ƿ��,ͨ����Һ©������1 mol��L-1ϡ����40 mL,�ռ�10 mL����,ͨ����¼�������������õ���Ӧ����Ϊx mol��(L��min)-1��ʵ�鿪ʼʱ����װ�������Եķ�������������������������������
(2)��ͬѧ��ͼ2װ�òⶨNa2CO3��NaCl�Ĺ���������Na2CO3����������:
���ס������Թܸ�����������,�������Ӷ�Ӧ�ӿں�,����ʢϡ������Թ�,������Ӧ,�ų�����,����������ϡ����Ӧ�ֱ�����������������������(���������);
��G�ܿ����û�ѧʵ�������һ�ֳ������������,����������������;
�������ס��ҽӿڵ����ӷ�ʽ����:A��������,B����������,C����������(��д���ӿڵı��);
��Ϊ��߲�����ȷ��,�ռ��������,��װ�ö���ǰӦ���еIJ�����������������������������������������������������
(3)��ͬѧ���ͬѧʵ��Ŀ����ͬ:��ͼ3װ�òⶨ���ɵ�CO2������,����װ�ô�������ȱ��,�Ӷ�����ʵ�����,�����������ʹ�ⶨ�������ƫ�����Ҫԭ��������������������������������
���𰸡�(1)��Ӧʱ�� �ر�A������,��ע������������һ������,һ��ʱ����ɿ�����,�������ܻص�ԭλ,֤����©��,����©��
(2)���ס��ҡ�����ʽ�ζ��ܡ���D��E��F���������ƶ��ζ���,ʹ��������Һ����ƽ��ƫ��
(3)CO2�����л���ˮ����������е�CO2��ˮ��������������
��������(2)Ϊ��˳����ϡ���ᵹ����һ�Թ�,����Ӧ�������Թ�����ѹƽ��,�����������������,����ֻ��һ��,���Լ�������ķ���װ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ��ķ�Ӧԭ�������ӷ���ʽ��ʾ��ȷ����(����)
A. �����£�����Ȼ����ҺpH<7��֤��һˮ�ϰ������NH4+��2H2O===NH3��H2O��H3O��
B. ������������Һ��ȥþ���е���������2Al��2OH����2H2O===2AlO2-��3H2��
C. ��̼��������Һ����ˮ�����е��Ȼ���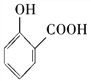 ��2HCO3-��
��2HCO3-�� ��2H2O��2CO2��
��2H2O��2CO2��
D. �ø�����ر���Һ�ζ����2MnO4-��16H����5C2O4-=2Mn2����10CO2����8H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��һ���¶��£���2 L�̶��ݻ����ܱ������з�����Ӧ��2N2O5(g)![]() 4NO2(g)+O2(g) ��H>0����Ӧ��Ͳ�������������ʵ����淴Ӧʱ��仯��������ͼ��ʾ��������ȷ���ǣ�
4NO2(g)+O2(g) ��H>0����Ӧ��Ͳ�������������ʵ����淴Ӧʱ��仯��������ͼ��ʾ��������ȷ���ǣ�

A. 0-20 s��ƽ����Ӧ����v(N2O5)=0.1 mol��L-1��s-1
B. 10 sʱ�������淴Ӧ������ȣ��ﵽƽ��
C. 20 sʱ������Ӧ���ʴ����淴Ӧ����
D. ����a��ʾNO2�����ʵ����淴Ӧʱ��ı仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ֱ�������Fe-Cu�Ͻ���Ʒ����������ΪFe2O3��CuO����8.0 g�������´�����

����˵����ȷ����
A����ҺA�е�������ΪFe2+��Fe3+��H+
B������Ʒ��Cu��OԪ�ص�������Ϊ10��l
C��V=448
D���ܽ���Ʒʱ����H2SO4�����ʵ���Ϊ0.04 mo1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪![]() ��
��![]() ��Һ��
��Һ��![]() ��Ũ��Ϊ
��Ũ��Ϊ![]() ����ش��������⣺
����ش��������⣺
��1�����������ܵ������__________�����ڵ���ʵ���__________�����ڷǵ���ʵ���__________��������ţ�
���Ȼ��ƾ��� �ڰ��� ��ˮ�� ������ ��ʯī ������![]() �߿��� ��BaSO4 ��ϡ����
�߿��� ��BaSO4 ��ϡ����
��2��д��![]() ��ˮ�ܽ��еĵ��뷽��ʽ��_________________��д��NaHSO4������״̬�µĵ��뷽��ʽ��_______________________________��
��ˮ�ܽ��еĵ��뷽��ʽ��_________________��д��NaHSO4������״̬�µĵ��뷽��ʽ��_______________________________��
��3��![]() ��Һ��NaHCO3��Һ������������ɣ���Ӧ�����ӷ���ʽ_______________
��Һ��NaHCO3��Һ������������ɣ���Ӧ�����ӷ���ʽ_______________
��4������![]() ��
��![]() ��Һ��Ϻ���Һ�����ԣ���д����Ӧ�����ӷ���ʽ_________________________________________________________________��
��Һ��Ϻ���Һ�����ԣ���д����Ӧ�����ӷ���ʽ_________________________________________________________________��
����![]() ��
��![]() ��Һ�л����Һ�ʼ��ԣ���д����Ӧ�����ӷ���ʽ___________________________________________________��
��Һ�л����Һ�ʼ��ԣ���д����Ӧ�����ӷ���ʽ___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����6�۸��Ļ����ﶾ�Խϴ���NaHSO3����Һ�е�Cr2O![]() ��ԭ��Cr3+���÷�Ӧ�����ӷ���ʽΪ__________________________________________________________��
��ԭ��Cr3+���÷�Ӧ�����ӷ���ʽΪ__________________________________________________________��
��2����ʢ��H2O2��Һ���Թ��м��뼸���ữ��FeCl2��Һ����Һ����ػ�ɫ��������Ӧ�����ӷ���ʽΪ____________________________________��
��3�� �������ʱV2O5ת��ΪVO![]() ����Ӧ�����ӷ���ʽΪ________________________________________��
����Ӧ�����ӷ���ʽΪ________________________________________��
��4����H2SO4�����Ի�����ClO2��⻯�ط�Ӧ�����ӷ���ʽ___________________________��
��5����֪������������NaClO2�ɷ�����Ӧ����NaCl���ͷų�ClO2���÷�Ӧ�����ӷ���ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ����ѧʵ���ڻ�ѧѧϰ�о��м�����Ҫ������.
(1)�����й�ʵ�����������ȷ����_______.
(A)����500mLij���ʵ���Ũ�ȵ���Һ��������ֻ250mL������ƿ
(B)����������������л��е�NaCl����
(C)���÷�Һ©�������ͺ�ˮ�Ļ��Һ�����
(D)����ʽ�ζ�����ȡ20. 00mL���������Һ
(E)Ϊ�˲ⶨij��Һ��pH������ˮ��ʪ��pH��ֽ���뵽������Һ����һ��ȡ���������ɫ�����жԱ�
(F)��Ũ��ˮϴ������������Ӧ���Թ�
(G)����������Һʱ����ϡ��ˮ�����μӵ���������Һ�У���������������μӵ������պ��ܽ�Ϊֹ
(H)����һ��Ũ�ȵ���Һʱ��������ʱ��С�ļ�ˮ��������ƿ�Ŀ̶��ߣ�Ӧ�����õι���ȥ����IJ��֡�
(J)�������������������������������
(K)�ýᾧ�����Գ�ȥ������л��е������Ȼ���
(2)��ͼΪ���������IJ��ֽṹ(�е��������Ŵ�)��
Aͼ��Һ����ʾ��Һ�����Ϊ__________mL�����������������е�ij�ֲ���һҺ��������ƽ��ʱ����ΪNmL������ʱ����ΪMmL����M>N������ʹ�õ�������___________(����ĸ���)��
(3)��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
����
����ʽ��HCl
��Է�����: 36.5
�ܶȣ�1.2g/ml
HCl����������36.5%
�ٸ�Ũ������HCl�����ʵ���Ũ��Ϊ_________mol/L��
��ijѧ����������Ũ���������ˮ����500 mL���ʵ���Ũ��Ϊ0.3 mol/Lϡ���ᡣ
��.��ѧ����Ҫ��ȡ________mL����Ũ����������ơ�
II.�����ƹ����У�����ʵ�������ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���______��
A.����Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B.��Һע������ƿǰû�лָ������¾ͽ��ж���
C.����ʱ���ӿ̶���
D.�����Dǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��C�׳ƿ������ᣬ��һ����Ҫ�Ļ���ԭ�ϣ�F�ǿ�����ҩ����ƽ��һ����Ҫ���м��壬���ǿ�������·�ߺϳɣ�

��֪��![]() (R��R��������ԭ�ӻ����)
(R��R��������ԭ�ӻ����)
(1)A��������_________��C��D�ķ�Ӧ������________________��
(2)C�еĺ���������������_______________��
(3)���й���C��E��˵����ȷ����__________��
a.�����ڷ����廯���� b.���ܷ���ȡ����Ӧ
c.����������������ȫ��ͬ d.������������Һ�ж��ܷ���ˮ�ⷴӦ
(4)д��E������������Һ�м���ˮ��Ļ�ѧ����ʽ_____________________________��
(5)д����C���ɸ߾���P�Ļ�ѧ����ʽ_____________________��
(6)ͬʱ��������������C��ͬ���칹�干��_______�֡�
����Ũ��ˮ�����а�ɫ�������ɣ�
�ڼ��ܷ���ˮ�ⷴӦҲ�ܷ���������Ӧ��
��Щͬ���칹���У����ڶ�ȡ�����Һ˴Ź���������ʾ5�����շ��ͬ���칹��Ľṹ��ʽ��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ȼ���ͭ��CuCl)�ǰ�ɫ��ĩ������ˮ������ϡ���ᷴӦ���㷺Ӧ���ڻ�����ӡȾ����ҵ��ij�о���ѧϰС�����ȷֽ�CuC122H2O�Ʊ�CuCl,���������̽������֪����������Cu+���ȶ�����

����˵����ȷ����
A. X���������N2��Ŀ������������������CuCl22H2O���ȹ��̿��ܵ�ˮ��
B. CuCl��ϡ���ᷴӦ�����ӷ���ʽΪ��2Cu++4H++SO42-�T2Cu2++SO2��+2H2O
C. ;��1�в�����Cl2���Ի���ѭ�����ã�Ҳ����ͨ�뱥��CaCl2��Һ�г�ȥ
D. ;��2��200���·�Ӧ�Ļ�ѧ����ʽΪ��Cu2(OH)2Cl2![]() 2CuO + 2HCl
2CuO + 2HCl
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com