

��1��FexO��xֵ����ȷ��0.01��Ϊ____________��
��2�������е�Fen+�ֱ�ΪFe2+��Fe3+,��Fe2+��Fe3+�������У�Fe2+��ռ��������С����ʾ����ȷ��0.001��Ϊ____________��
��3���˾��廯ѧʽΪ____________��
��4����ij��Fe2+(��Fe3+)��������ҵȾ����O2-Χ�ɵĿռ伸����״��____________��
��5���ھ����У���Ԫ�ص����Ӽ���̾���Ϊ____________m��
��������1����NaCl����ṹ��֪��1��NaCl������8��С�����幹�ɣ�ÿ��С�������8������ֱ���4��Na+��4��Cl-����ռ�ݣ�ÿ��Na+��Cl-��ֻ��1/8ռ����С�������У����ÿ��С�����庬Na+(1/8)��4��=1/2��,��Cl-Ϊ(1/8)��4��=1/2��������NaCl������8��С��������ɣ���ÿ��������NaCl����8��(1/2)=4����ͬ������FexO��������4��FexO������1 mol��������4 mol FexO���ӡ���FexO��Ħ������ΪM�����У�4M=��VNa��M=��VNa/4=\/4=67.4 g��mol-1��
��FexO��x��55.9 g��mol-1+16 g��mol-1=67.4 g��mol-1����x=0.92��
��2���躬y��Fe2+������(0.92��y)��Fe3+�������������ϼ۴�����Ϊ�㣬�ã�
2y+3(0.92��y)=2�����y=0.76������Fe2+��ռ����Ϊ:0.76/0.92=0.826��
��3������Fe2+Ϊ0.76����Fe3+Ϊ(0.92��0.76)=0.16���ʻ�ѧʽΪ:![]() ��
��
��4����NaCl����ṹ����֪����Fe2+(��Fe3+)��������ҵȾ����O2-��6������6��O2-��Χ�ɵļ�����״Ϊ�������塣

��5����FexO��Fe��Fe��̵ľ���Ϊr����FexO�����һ��ƽ��ͼ��֪����ֱ��������ABC�У�б��BCΪr����AB=AC=�����߳�/2��
���r2=AB2+AC2,
r=![]() =3.03��10-10m��
=3.03��10-10m��
�𰸣���1��0.92 ��2��0.826 ��3��![]() (4)�������� ��5��3.03��10-10
(4)�������� ��5��3.03��10-10


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ���������ѧУ2011��2012ѧ��߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�022
(1)�۵�е�HF________HI��ԭ��________��
(2)���ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ________��
(3)����4�������۵�е��ɸߵ�������Ϊ________(�����)��
�ٽ��ʯ(C��C)����(Ge��Ge)
�۾����(Si��Si)�ܽ��ɰ(Si��C)
(4)������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ����ͼ��ʾ��������ÿ��Na��ͬʱ������________��Cl��������ЩCl�����ɵļ��ι���Ϊ________��������ÿ��Cl����Χ������ӽ��Ҿ�����ȵ�Cl������________����

(5)ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p34s1�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ________��������������Ӧ��ˮ����Ļ�ѧʽ��________��
(6)ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ�________���ڣ���________�壬________��Ԫ�أ�Ԫ�ط�����________����ԭ�Ӻ�����________���ܼ��������Ƶ���״��________�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ���������ѧУ�߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
��1�����۵�е�HF HI��ԭ��
��2�������ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ
��3��������4�������۵�е��ɸߵ�������Ϊ________ ������ţ�
�ٽ��ʯ��C��C�����ࣨGe��Ge��
�۾���裨Si��Si���ܽ��ɰ��Si��C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��
������ÿ��Na+ͬʱ������______��Cl-������Щ Cl-���ɵļ��ι���Ϊ ��������ÿ��Cl-��Χ������ӽ��Ҿ�����ȵ�Cl-����________����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s 2s
2s 2p
2p 3s
3s 3p
3p 4s
4s �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ ��������������Ӧ��ˮ����Ļ�ѧʽ��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ� ���ڣ��� �壬 ��Ԫ�أ�Ԫ�ط����� ����ԭ�Ӻ����� ���ܼ��������Ƶ���״�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�켪��ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
��1�����۵�е�HF HI��ԭ��
��2�������ֺ�����HClO��HClO3��H2SO3��HClO4��������ǿ��������Ϊ
��3��������4�������۵�е��ɸߵ�������Ϊ________ ������ţ�
�ٽ��ʯ��C��C�����ࣨGe��Ge��
�۾���裨Si��Si���ܽ��ɰ��Si��C��
��4��������й���ļ������Σ���������������ظ���Ԫ��֮Ϊ������NaCl����ṹ��ͼ��ʾ��

������ÿ��Na+ͬʱ������______��Cl-������Щ Cl-���ɵļ��ι���Ϊ ��������ÿ��Cl-��Χ������ӽ��Ҿ����� �ȵ�Cl-����________����
��5��ijԪ�صļ���̬ԭ�ӵĵ����Ų�ʽΪ1s 2s
2s 2p
2p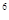 3s
3s 3p
3p 4s
4s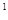 �����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
�����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ
��������������Ӧ��ˮ����Ļ�ѧʽ��
��6��ijԪ��ԭ�ӵļ۵��ӹ���Ϊ3d104S2�������ڵ� ���ڣ��� �壬 ��Ԫ�أ�Ԫ�ط����� ����ԭ�Ӻ����� ���ܼ��������Ƶ���״�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com