
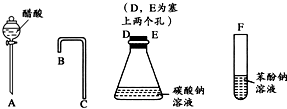
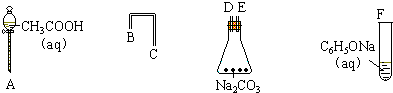
 ���һ����һ�������ʵ���װ��ͼ����֤������Һ��������̼ˮ��Һ��������Һ������ǿ��˳����CH3COOH��H2CO3��C6H5OH��
���һ����һ�������ʵ���װ��ͼ����֤������Һ��������̼ˮ��Һ��������Һ������ǿ��˳����CH3COOH��H2CO3��C6H5OH�� ˵������H2CO3��
˵������H2CO3��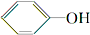 ��
��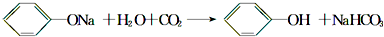 ��
��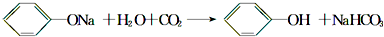 ��
��

 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͼ6-18
(1)����ͼ6-18��ʾ����������װʵ��װ�ã�������������˳��Ϊ��___________��___________��___________��___________��___________��___________��
(2)д��ʵ��������йصĻ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
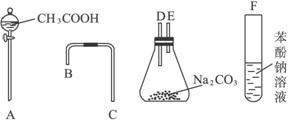
���һ����һ�������ʵ���װ��ͼ��Ŀ������֤������Һ��������̼ˮ��Һ��������Һ������ǿ��˳��
�� ������ͼ��ʾ������������װʵ��װ�ã�������������˳��Ϊ�� �� �� �� ������ĸ��
�� д��װ��I��II�е�ʵ������
I�� ��
II�� ��
�� д��װ��I��װ��II�з�����Ӧ�Ļ�ѧ����ʽ
I�� ��
II�� ��
����Ϊ��ʵ�鲻���ʵĵط���_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�ϸ߶��и߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ������
Ϊ��ȷ��CH3COOH , OH��H2CO3������ǿ�����������һ����һ�������ʵ���װ��ͼ������ʾ����ÿ��2�ֹ�8�֣�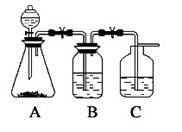
(1)����ƿ��װ��һ������ˮ�����ι��壬��A�з�����Ӧ�����ӷ���ʽΪ
��2��װ��B��ʢ�ŵ��Լ��� ������������
��3��ʵ���й۲쵽C�г��ֵ���Ҫ������
.
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com