
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�
| �� | |||||||||||||||||
| �� | �� | ||||||||||||||||
| �� | �� | �� | �� | ||||||||||||||
| �� | �� | �� |
��ش��������⣺
��1����������d����Ԫ���� ����Ԫ�ط��ţ����۵��ӣ���Χ���ӣ��Ų�ʽΪ ��
��2���� �� �� ������Ԫ�صĵ�һ�������ɴ�С��˳���� ����Ԫ�ط��ű�ʾ����
��3����ԭ�ӹ�����ص���ʽ��������γɵĻ������У����Т۵��ӻ���ʽΪ ��
��4��ijԪ�ص����������Ų�ʽΪnsnnpn+1����Ԫ��ԭ�ӵĺ����������ӵŶԵ�����Ϊ ����Ԫ����Ԫ�آ��γɵ�10���ӷ���X���� ���ӣ�����ԡ��Ǽ��ԡ�����
��5��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʡ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��6��1183 K���¢�Ԫ���γɵľ���Ļ����ṹ��Ԫ��ͼ1��ʾ��1183 K����ת��Ϊͼ2��ʾ�ṹ�Ļ����ṹ��Ԫ�������־��������ڽ���ԭ�Ӽ������ͬ��
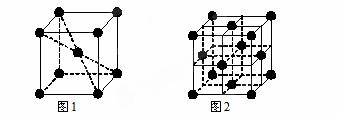
��1183 K���µľ����У����ԭ�ӵȾ���������Ģ�ԭ����Ϊ______������1183 K���ϵľ����У����ԭ�ӵȾ���������Ģ�ԭ����Ϊ________��
 ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ���������������A��B��C��D��E��F��G����ǰ������Ԫ�أ�ϡ��������⣩�������£�������Ԫ�ض�Ӧ�ĵ��ʳ���̬��C��Dͬ�塣����Aԭ�Ӻ��������ֲ�ͬ�˶�״̬�ĵ��ӣ�CΪ�ؿ��к��������Ԫ�أ�F�ļ�������ͬ��������Ԫ���γɵļ������������������ģ�G�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ�
��1���縺��Խ���ԭ�ӣ��Լ��ϵ��ӵ���������Խǿ��ͨ����ӦԪ�ػ��ϼ۳��ָ��ۡ�����B��C��D��E����Ԫ���γɵij���������Ļ��ϼۣ�ȷ���縺������Ԫ�أ�д�����̬ԭ�ӵĵ����Ų�ͼ ��
��2��������BԪ�صȷǽ���Ԫ����ɵ����ӻ��������E���⻯����Һ����������Һ��Ӧ���ɣ�д���÷�Ӧ�����ӷ���ʽ ��
��3��AԪ�ؿ��γɶ��ֵ��ʣ�һ�־���ṹ��ͼһ��ʾ���þ��������ڵ�F��������ã��γ�ij����ͭɫ�����ʣ����е�Ԫ��F�á���ʾ����ԭ�ӷֲ���ͼ����ʾ�������ʵĻ�ѧʽΪ ����һ�ֵľ�����ͼ����ʾ�����˾����е��ⳤΪ356.6 pm����˾������ܶ�Ϊ___________g��cm-3(������λ��Ч����)��
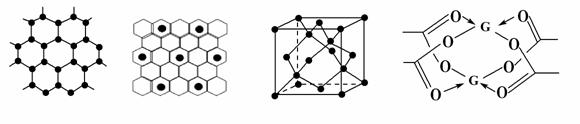 ͼһ���� ������ ͼ������ �������� ͼ�� ͼ��
ͼһ���� ������ ͼ������ �������� ͼ�� ͼ��
��4��GԪ���γɵĵ��ʣ��侧��Ķѻ�ģ��Ϊ________��G�Ĵ����ξ���ֲ��ṹ��ͼ�ģ��þ����к��еĻ�ѧ����________(��ѡ�����)��
�ټ��Լ������ڷǼ��Լ���������λ�������ܽ�����
��5��E��F�벻ͬ��̬��G��������ֻ���������ֻ����ﶼ�����ڴ���Ȳ�ۺϣ��������Ӿ�����G��E��Ԫ���γɵ��������ṹ(����ͼ)����֪����һ�ֻ�����Ļ�ѧʽΪFGE3����һ�ֵĻ�ѧʽΪ ��
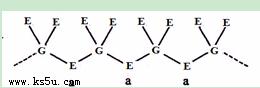
��6��������F2D3��һ�ֺ��зǼ��Թ��ۼ������ӻ������ԭ������㶼����8e-�ȶ��ṹ������д���û����������ӵĵ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȡ����п�ۣ��ֱ�ʢ�ڼס��ҡ�����֧�Թ��У�������Ҫ���������ʺ��������ӣ���ʱ�ⶨ�������������������50 mL pH��3�����ᣬ�Ҽ���50 mL pH��3�Ĵ��ᣬ������50 mL pH��3�Ĵ��ἰ����������ĩ������Ӧ���ˣ��������������һ���࣬��û��ʣ���п�����á����������������ش����и��⡣
(1)��ʼʱ����Ӧ���ʵĴ�СΪ ��
(2)��֧�Թ��вμӷ�Ӧ��п������Ϊ ��
(3)��Ӧ���ˣ�����ʱ��Ϊ ��
(4)�ڷ�Ӧ�����У��ҡ������ʲ�ͬ��������(��Ҫ˵��) ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���� ��������
A��1 mol��L��1NaCl��Һ����NA��Na��
B��10 mL��������Ϊ98%��H2SO4��ˮϡ����100 mL��H2SO4����������Ӧ����9.8%
C������240 mL 1 mol��L��1��NaOH��Һ���NaOH���������Ϊ9.6 g
D������1 mol��L��1��H2SO4��Һʱ������ȡ��ŨH2SO4��������ƿ�м�ˮϡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�������ȡ�ķ��뷽�����Ӧԭ������ȷ���� ��������
| ѡ�� | Ŀ�� | ���뷽�� | ԭ�� |
| A | ��������ˮ�еĵ� | �Ҵ���ȡ | �����Ҵ��е��ܽ�Ƚϴ� |
| B | ���������������Ҵ� | ��Һ | �����������Ҵ����ܶȲ�ͬ |
| C | ��ȥKNO3�л��ӵ�NaCl | �ؽᾧ | �Ȼ�����ˮ�е��ܽ�Ⱥܴ� |
| D | ��ȥ�����е����� | ���� | ���������ѵķе����ϴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڻ�ѧ����ı�ʾ��ȷ����( )
A���������Ƶĵ���ʽ�� B��������Ϊ35��������Ϊ45����ԭ�ӣ�
B��������Ϊ35��������Ϊ45����ԭ�ӣ� Br
Br
C�������ӵĽṹʾ��ͼ�� D��HClO�Ľṹʽ��H��Cl��O
D��HClO�Ľṹʽ��H��Cl��O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2A+B 3C+4D��Ӧ�У������ʾ�Ļ�ѧ��Ӧ����������(�� ��)
A��v(A) = 0.5mol/(L��min) B��v(B) = 0.05mol/(L��s)
C��v(C) = 0.9mol/(L��min) D��v(D) = 1.0mol/(L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��E����Ԫ�ؾ�Ϊ������Ԫ�أ�ԭ������������AԪ��ԭ�ӵĺ�������������Ӳ�������������������ȡ�B��D��E����Ԫ�������ڱ��е����λ����ͼ����ʾ��ֻ��EԪ�صĵ�������ˮ��Ӧ���������ᡣ�ס��ҡ�M��W��X��Y��Z�������ʾ���A��B��D����Ԫ���е�һ�ֻ�����ɣ�����ֻ��M����ͬʱ��������Ԫ�أ�WΪA��B��Ԫ����ɵ�10���ӷ��ӣ�����ˮ�Լ��ԣ��ס���Ϊ�ǽ������ʣ�X���Ӻ���10�����ӣ�������Ϊ��ɫҺ�塣����֮���ת����ϵ��ͼ����ʾ��
| B | D | |
| E |
ͼ��

��ش��������⣺
(1) Z�Ļ�ѧʽΪ__________________
(2)�ҵĵ���ʽΪ__________________��
(3)���Ե缫���NaE��Һ�����ӷ���ʽΪ__________________________��
(4)W���Ӧ����ʽΪ__________________��
(5)��һ������A2��B2�Ļ���������1 L�ܱ������У���500 �桢2��107 Pa�´ﵽƽ�⡣���ƽ������������ʵ���Ϊ0.50 mol������A2Ϊ0.3 mol��B2Ϊ0.1 mol�����������A2��ƽ��ת����Ϊ________�����¶��µ�ƽ�ⳣ��Ϊ________(�������3λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
±�������������ƴ��ڵ�������ˮ�⣬����һ�����͵�ȡ����Ӧ����ʵ���Ǵ������ԭ���ţ���OH-�������ӣ�ȡ����±�����е�±ԭ�ӡ�����CH3CH2CH2��Br+OH- CH3CH2CH2��OH+Br-��
CH3CH2CH2��OH+Br-��
д�����з�Ӧ�Ļ�ѧ����ʽ��
��1���������NaHS��Ӧ______________________________��
��2��������CH3COONa��Ӧ____________________________________________��
��3���ɵ���顢��ˮ�Ҵ��ͽ����ƺϳɼ����ѣ�CH3OCH2CH3��______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com