
| V |
| Vm |
| m |
| n |
| 5.6L |
| 22.4L/mol |
| 0.04mol��1 |
| 0.02mol |
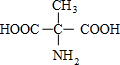 ��
��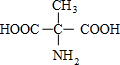 ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH3Cl |
| B��CH2Cl2 |
| C��CCl4 |
| D��CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������������Դ���������й㷺��;��������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4��װ����ͼ��ʾ�������ƶϺ������ǣ�������
������������Դ���������й㷺��;��������Ni���������缫���ŨNaOH��Һ�Ʊ���������Na2FeO4��װ����ͼ��ʾ�������ƶϺ������ǣ�������| A�������������缫��ӦΪFe-6e-+4H2O�TFeO42-+8H+ |
| B�����缫�ϵĵ缫��ӦΪ2H2O+2e-�TH2��+2OH- |
| C������ĤΪ�����ӽ���Ĥ����OH-���������ƶ� |
| D�����ʱ������pH���͡�������pH���ߣ�������ҺpH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�� |
B�� |
C�� |
D�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 H2O2����Ҫ��������������ˮ��Һ������������ɱ����Ư�ȣ���ش������й����⣮
H2O2����Ҫ��������������ˮ��Һ������������ɱ����Ư�ȣ���ش������й����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com