
���������ֻ��ţ��ٱ��� ��ȩ�� ���ǻ� ���Ȼ� ���һ� ����ԭ�ӣ���Cl�������ֻ���������ϣ����γ��µĻ����д���������������Ļ�����Ľṹ��ʽ��
��1����������ԭ����������2�����������ԣ������ܺ�̼�����Ʒ�Ӧ ��
��2����������ԭ����������2�����ܷ���������Ӧ�����ʣ�������ȩ�ͼ����ȣ�Cl-CHO����� �� ��
��3�����ȶ�����Ԫ���� ��
��֪ʶ�㡿�л���ѧ�����ŵ�����
���𰸽�������1��C6H5OH ��2��HCOOH CH3CH2CHO ��3��HOCOOH
��������1����������ԭ����������2�����������ԣ������ܺ�̼�����Ʒ�Ӧ�������Ȼ���ֻ
�ܺ��з��ǻ��������ӣ��ṹ��ʽΪ��C6H5OH����2����������ԭ����������2�����ܷ���������Ӧ������ȩ����������ȩ�ͼ����ȣ�Cl-CHO����Т�ȩ������ǻ����ϵ�HCOOH����ȩ������һ���ϵ�CH3CH2CHO����3�����ȶ�����Ԫ������HOCOOH
��˼·�㲦�����⿼���л���Ľṹ��ʽ��д���ؼ������ղ�ͬ�����ŵ����ʡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����2011��6��1���������ͽ����������棬���ܻ�������ʳƷ�п���
Υ�����ӵķ�ʳ�����ʺ������õ�ʳƷ���Ӽ������������ںų����µ�һ
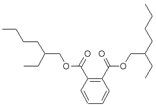
�Ƶľƹ��Ʊ����ܻ�����������ߴ�260%,��Ӱ�췢���͵��¸ΰ�����֪�ܻ���DEHP���ӽṹ����ͼ������˵��������ȷ���� ( )
A��DEHP�Ļ�ѧʽΪ��C24H38O4
B��DEHP���������ʣ�������ˮ
C��DEHP������������Һ�������ữ�ɵ������л�����
D��DEHP����ʹ��ˮ������ѧ��Ӧ����ɫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A������������Ħ�����������
B��18 gˮ�к���1 molˮ
C��O2��Ħ������(��λ��g��mol��1)����ֵ�ϵ�������Է�������
D��1 mol CO��������28 g��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���н�������A��B������ס��ҡ����Լ�����C��D��E��F������֮���ܷ������·�Ӧ����ͼ����Щ��Ӧ�IJ���ͷ�Ӧ����û�б����

��ش��������⣺
��1��A�Ļ�ѧʽΪ �����Ļ�ѧʽΪ ��
��2��д�����з�Ӧ�����ӷ���ʽ��
�� ��
�� ��
�� ��
��3��д��C��Һ��Al��Ӧ�Ļ�ѧ����ʽ�� ��
��4����Na2O2Ͷ�뵽E��Һ�У����Թ۲쵽�������ǣ� ��
��5��Ϊ����B���ʣ�����������ϡ���ᣬȡ�ϲ���Һ�����ټ�����Լ�����д��ĸ���ţ��� ��
a. ��ˮ b. ��ˮ c. NaOH��Һ d. KSCN��Һ e. Na2SO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A�Ƿ������ĺ��������Ϊ�˲ⶨ�������ɣ���������ʵ�飺
����һ���¶Ⱥ�ѹǿ�½�A�������������������ͬ�¡�ͬѹ�µ��������������38����
�ڳ�ȡ7.6gA����11.2L��������ȫȼ�գ������ֻ��CO2��ˮ����������Ӧ��Ļ���� ͨ
��Ũ��������Ϊ10.64L��ŨH2SO4��������3.6g���ٽ����µ�����ͨ��ʢ������Na2O2�ĸ���ܺ��������������6.16L������������ڱ�״���²ⶨ�����Իش�
��1��A�ķ���ʽΪ ��
��2��A���ʱ�����ֻ��һȡ��������FeCl3��Һ����ɫ��Ӧ��1molAֻ����1molNaHCO3��Ӧ��1molA������Na��Ӧ����1molH2����A�Ľṹ��ʽΪ ��
��3��A�������Ʒ�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������������������Ʊ��������������й����ʵ�����������±���
| ������ | ��Է������� | �ܶ�/g·cm-3 | �е�/�� | �ܽ��/100gˮ |
| ������ | 74 | 0.80 | 118.0 | 9 |
| ������ | 60 | 1.045 | 118.1 | ���� |
| ���������� | 116 | 0.882 | 126.1 | 0.7 |
��һ���������������Ʊ�
���ڸ����50mLԲ����ƿ�У�����13.5mL��������7.2mL�����ᣬ�ټ���3��4��Ũ���ᣬҡ�ȣ�Ͷ��1��2����ʯ��Ȼ��װ��ˮ��(���ã�ʵ������в��Ϸ����ȥ��Ӧ���ɵ�ˮ)���¶ȼƼ����������ܣ���������������Ӧ
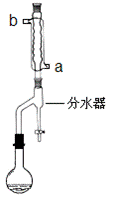
��������Ʒ�ľ���
�ڽ���ˮ���ֳ�������ͷ�ӦҺһ�����Һ©���У���10 mL��ˮϴ�ӡ��л��������10 mL10%Na2CO3ϴ�������ԣ�����10 mL ��ˮϴ�ӣ�����л���ת������ƿ�У�������ˮ����þ���
�۽���������������������50 mL ��ƿ�У���ѹ�����ռ�125��127 �����֣���11.6 g����������
��ش��й����⡣
��1����ˮӦ�ô������� ___________����a��b���ܿ�ͨ�롣
��2��������в��ϴӷ�ˮ���²��ֳ����ɵ�ˮ��Ŀ����_________________________
��������жϷ�Ӧ�յ��������_____________________________________��
��3����Ʒ�ľ��ƹ��̲�����У���һ��ˮϴ��Ŀ����_____________________________���ñ���Na2CO3��Һϴ���л��㣬�ò�������Ŀ����_________________________________��
��4�����й��ڷ�Һ©����ʹ��������ȷ����____
A����Һ©��ʹ��ǰ����Ҫ��©��ֻҪ��Һ©��������о����©ˮ����ʹ��
B��װҺʱ����Һ©����Һ�����������ó������ݻ���2/3
C����ȡ����Ӧ����ͼ��ʾ
D���ų�Һ��ʱ���轫��������ʹ���ϵİ��۶�©�����ϵ�С��
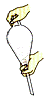
��5������۵ij�ѹ�������ռ�126�����֣��е����140����л������������һ�㲻�����������ܶ��ÿ��������ܣ�����ԭ����_________________________
��6����ʵ������У�����������������ʽ��116���IJ�����__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ij�л��� ������������ȷ���� ��������
������������ȷ���� ��������
A��1 mol���л��������3 mol Na������Ӧ��
B��1 mol���л��������3 mol NaOH������Ӧ
C��1 mol���л��������6 mol H2�����ӳɷ�Ӧ
D��1 mol���л���ֱ�������Na��NaHCO3��Ӧ����������������ͬ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������е����Ų�ʽ��ԭ���У��뾶������ �� ��
A ls22s22p63s23p5 B 1s22s22p63s23p2 C 1s22s22p2 D 1s22s22p63s23p4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NH3������NO����Ⱦ����Ӧ����ʽΪ��6NO��4NH3 �� 5N2��6H2O������NO��NH3�Ļ����1 mol��ַ�Ӧ������ԭ��������������1.4 g���������ж�����ȷ���ǣ� ��
A����������Ϊ5.6 L B����0.3 mol NO������
C��������������2.8 g D��ԭ�������NO��NH3�����ʵ���֮�ȿ���Ϊ3 : 2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com