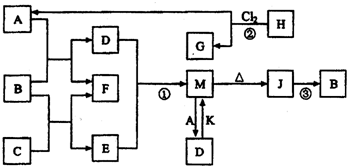
��2012?�Ͽ���һģ��ͼ��B��FΪ���ʣ�����Ϊ���������֮���������ת����ϵ�����ַ�Ӧ������P��Ӧ��������ȥ�������У�A��C�ǹ�ҵ����;�ܹ��������Ҫ����ԭ�ϣ�C����ɫ��ӦΪ��ɫ��BΪ�ճ������г����Ľ�����H��G���Ӿ�������������ṹ��H��һ����Ҫ����Դ��J��һ�ֽϺõ��ͻ���ϣ�K�Ǽ�������ˮ�ļ������壬��ش��������⣺
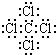
��1��C�д��ڵĻ�ѧ������Ϊ
���Ӽ����ۼ�
���Ӽ����ۼ�
��G�ĵ���ʽΪ
��
��2����Ӧ�ڽ��е�����
���ա���������
���ա���������
��
��3����Ӧ�ٵ����ӷ���ʽΪ
Al3++3AlO2-+6H2O=4Al��OH��3��
Al3++3AlO2-+6H2O=4Al��OH��3��
��
��Ӧ�۵Ļ�ѧ����ʽΪΪ
��
��4����D��Һ��ͨ��K������M����һ������X��д������X�������ӵ�ʵ�鷽��������Ϊ
ȡ����X��Һ���Թ��У��ý�ͷ�ιܵ���NaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������Թܣ��ɹ۲쵽��ɫʯ����ֽ����
ȡ����X��Һ���Թ��У��ý�ͷ�ιܵ���NaOH��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ������Թܣ��ɹ۲쵽��ɫʯ����ֽ����
��
��5������ȵ�ԭ������֮��Ϊ36��������131��ԭ�Ӻ���������Ϊ
78
78
������Ԫ�����ڱ��е�λ��Ϊ
�������ڵڢ�A��
�������ڵڢ�A��
��
��6���ҹ��״��ĺ�������B����Ϊ����������Ϊ��������ˮΪ�������Һ�������е�������B��Ӧ�����������õ�ص�������ӦʽΪ
O2+2H2O+4e-=4OH-����3O2+6H2O+12e-=12OH-��
O2+2H2O+4e-=4OH-����3O2+6H2O+12e-=12OH-��
��




 ��1��C�д��ڵĻ�ѧ������Ϊ
��1��C�д��ڵĻ�ѧ������Ϊ