
1000”ꏱ£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO4¹ĢĢåŹ¹Ęä·¢ÉśŅŌĻĀ·“Ó¦²¢“ļĘ½ŗā£ŗNa2SO4(s)+4H2(g) Na2S(s)+4H2O(g)£¬”÷H<0£¬ŌŚŗćĪĀŗćČŻŹ±»Ų“šŅŌĻĀĪŹĢā£ŗ
Na2S(s)+4H2O(g)£¬”÷H<0£¬ŌŚŗćĪĀŗćČŻŹ±»Ų“šŅŌĻĀĪŹĢā£ŗ
(1)ĻņČŻĘ÷ÖŠ·Ö±š¼ÓČėŅŌĻĀĪļÖŹ£¬ÅŠ¶Ļ¶ŌĘ½ŗāÓŠĪŽÓ°Ļģ£¬ÓŠÓ°ĻģµÄÓ¦ĢīŠ“³öĘ½ŗāŅĘ¶ÆµÄ·½Ļņ”£
¢Ł¼ÓČėNa2SO4_________________”£¢Ś¼ÓČė½¹Ģæ________________”£
(2)Čō³õŹ±¼ÓČėµÄNa2SO4ŹĒ1£®42g£¬Ę½ŗāŹ±ČŻĘ÷ÖŠ¹ĢĢåĪļÖŹÖŹĮæŹĒ1£®10g£¬Na2SO4µÄ×Ŗ»ÆĀŹŹĒ_______________”£
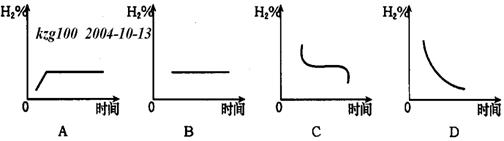
(3)Čō½«ČŻĘ÷ĪĀ¶ČÉżøß20”ę£¬H2ŌŚ»ģŗĻĘųĢåÖŠŗ¬Įæ±ä»ÆÓĆĻĀĶ¼ÖŠ_____ Ķ¼±ķŹ¾×īŗĻŹŹ”£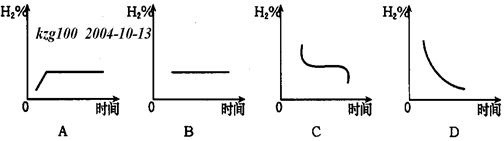

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij°ą¼¶Ķ¬Ń§²éŌÄ׏ĮĻ£¬¶ŌNa2SÓŠĮĖŅ»¶ØµÄČĻŹ¶£¬²¢Ģį³öĮĖŅŌĻĀ¼øøöĪŹĢā£¬Ēė»Ų“š£ŗ
(1)1000”ꏱ£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO4¹ĢĢåŹ¹Ęä·¢ÉśŅŌĻĀ·“Ó¦“ļµ½Ę½ŗā£ŗ
Na2SO4(s)£«4H2(g) ![]() Na2S(s)£«4H2O(g)””¦¤H>0Čō·“Ó¦ŌŚŗćĪĀŗćȯדĢ¬ĻĀ½ųŠŠ£¬»Ų“šŅŌĻĀĪŹĢā£ŗ
Na2S(s)£«4H2O(g)””¦¤H>0Čō·“Ó¦ŌŚŗćĪĀŗćȯדĢ¬ĻĀ½ųŠŠ£¬»Ų“šŅŌĻĀĪŹĢā£ŗ
ĻņČŻĘ÷ÖŠ·Ö±š¼ÓČėŅŌĻĀĪļÖŹ£¬ÅŠ¶Ļ¶ŌĘ½ŗāÓŠĪŽÓ°Ļģ£¬ÓŠÓ°ĻģµÄĢīŠ“³öĘ½ŗāŅĘ¶ÆµÄ·½Ļņ”£
¢Ł¼ÓČėNa2SO4”””””””””””£¢Ś¼ÓČė½¹Ģæ”””””””””””””””””””””””£
(2)ĻņNa2SµÄÅØČÜŅŗÖŠÖšµĪ¼ÓČėĻ”ŃĪĖį£¬Ö±µ½²»ŌŁÉś³ÉH2SĘųĢåĪŖÖ¹£¬ŌņŌŚ“Ė¹ż³ĢÖŠ£¬ČÜŅŗµÄc(HS£)±ä»ÆĒ÷ŹĘæÉÄÜŹĒ”””””””£
a.Öš½„¼õŠ” b.Öš½„Ōö“ó
c.ĻČÖš½„Ōö“󣬶ųŗó¼õŠ” d.ĻČÖš½„¼õŠ”£¬¶ųŗóŌö“ó
(3)Na2SČÜŅŗÓėĻĀĮŠČÜŅŗ»ģŗĻ£¬²»ÄÜ·¢Éś·“Ó¦µÄŹĒ”””””””””£
¢ŁH2S””¢ŚSO2””¢ŪNa2SO3””¢ÜĖįŠŌKMnO4ČÜŅŗ””¢ŻCuSO4 ¢ŽĀČĖ®
(4)Š“³öNa2SŗĶAlCl3ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij°ą¼¶Ķ¬Ń§²éŌÄ׏ĮĻ£¬¶ŌNa2SÓŠĮĖŅ»¶ØµÄČĻŹ¶£¬²¢Ģį³öĮĖŅŌĻĀ¼øøöĪŹĢā£¬Ēė»Ų“š£ŗ
(1)1000”ꏱ£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO4¹ĢĢåŹ¹Ęä·¢ÉśŅŌĻĀ·“Ó¦“ļµ½Ę½ŗā£ŗ
Na2SO4(s)£«4H2(g) Na2S(s)£«4H2O(g)””¦¤H>0Čō·“Ó¦ŌŚŗćĪĀŗćȯדĢ¬ĻĀ½ųŠŠ£¬»Ų“šŅŌĻĀĪŹĢā£ŗ
ĻņČŻĘ÷ÖŠ·Ö±š¼ÓČėŅŌĻĀĪļÖŹ£¬ÅŠ¶Ļ¶ŌĘ½ŗāÓŠĪŽÓ°Ļģ£¬ÓŠÓ°ĻģµÄĢīŠ“³öĘ½ŗāŅĘ¶ÆµÄ·½Ļņ”£
¢Ł¼ÓČėNa2SO4”””””””””””£¢Ś¼ÓČė½¹Ģæ”””””””””””””””””””””””£
(2)ĻņNa2SµÄÅØČÜŅŗÖŠÖšµĪ¼ÓČėĻ”ŃĪĖį£¬Ö±µ½²»ŌŁÉś³ÉH2SĘųĢåĪŖÖ¹£¬ŌņŌŚ“Ė¹ż³ĢÖŠ£¬ČÜŅŗµÄc(HS£)±ä»ÆĒ÷ŹĘæÉÄÜŹĒ”””””””£
a.Öš½„¼õŠ” b.Öš½„Ōö“ó
c.ĻČÖš½„Ōö“󣬶ųŗó¼õŠ” d.ĻČÖš½„¼õŠ”£¬¶ųŗóŌö“ó
(3)Na2SČÜŅŗÓėĻĀĮŠČÜŅŗ»ģŗĻ£¬²»ÄÜ·¢Éś·“Ó¦µÄŹĒ”””””””””£
¢ŁH2S””¢ŚSO2””¢ŪNa2SO3””¢ÜĖįŠŌKMnO4ČÜŅŗ””¢ŻCuSO4 ¢ŽĀČĖ®
(4)Š“³öNa2SŗĶAlCl3ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011½ģŗžÄĻŹ”³¤É³ŹŠŅ»ÖŠŃ§øßČżµŚĮł“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ij°ą¼¶Ķ¬Ń§²éŌÄ׏ĮĻ£¬¶ŌNa2SÓŠĮĖŅ»¶ØµÄČĻŹ¶£¬²¢Ģį³öĮĖŅŌĻĀ¼øøöĪŹĢā£¬Ēė»Ų“š£ŗ
(1)1000”ꏱ£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO4¹ĢĢåŹ¹Ęä·¢ÉśŅŌĻĀ·“Ó¦“ļµ½Ę½ŗā£ŗ
Na2SO4(s)£«4H2(g)  Na2S(s)£«4H2O(g)””¦¤H>0Čō·“Ó¦ŌŚŗćĪĀŗćȯדĢ¬ĻĀ½ųŠŠ£¬»Ų“šŅŌĻĀĪŹĢā£ŗ
Na2S(s)£«4H2O(g)””¦¤H>0Čō·“Ó¦ŌŚŗćĪĀŗćȯדĢ¬ĻĀ½ųŠŠ£¬»Ų“šŅŌĻĀĪŹĢā£ŗ
ĻņČŻĘ÷ÖŠ·Ö±š¼ÓČėŅŌĻĀĪļÖŹ£¬ÅŠ¶Ļ¶ŌĘ½ŗāÓŠĪŽÓ°Ļģ£¬ÓŠÓ°ĻģµÄĢīŠ“³öĘ½ŗāŅĘ¶ÆµÄ·½Ļņ”£
¢Ł¼ÓČėNa2SO4”””””””””””£¢Ś¼ÓČė½¹Ģæ”””””””””””””””””””””””£
(2)ĻņNa2SµÄÅØČÜŅŗÖŠÖšµĪ¼ÓČėĻ”ŃĪĖį£¬Ö±µ½²»ŌŁÉś³ÉH2SĘųĢåĪŖÖ¹£¬ŌņŌŚ“Ė¹ż³ĢÖŠ£¬ČÜŅŗµÄc(HS£)±ä»ÆĒ÷ŹĘæÉÄÜŹĒ”””””””£
a.Öš½„¼õŠ” b.Öš½„Ōö“ó
c.ĻČÖš½„Ōö“󣬶ųŗó¼õŠ” d.ĻČÖš½„¼õŠ”£¬¶ųŗóŌö“ó
(3)Na2SČÜŅŗÓėĻĀĮŠČÜŅŗ»ģŗĻ£¬²»ÄÜ·¢Éś·“Ó¦µÄŹĒ”””””””””£
¢ŁH2S””¢ŚSO2””¢ŪNa2SO3””¢ÜĖįŠŌKMnO4ČÜŅŗ””¢ŻCuSO4 ¢ŽĀČĖ®
(4)Š“³öNa2SŗĶAlCl3ČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģ°²»ÕŹ”ø߶žµŚŅ»“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
1000”ꏱ£¬ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČėŅ»¶ØĮæµÄNa2SO4¹ĢĢåŹ¹Ęä·¢ÉśŅŌĻĀ·“Ó¦²¢“ļĘ½ŗā£ŗNa2SO4(s)+4H2(g) Na2S(s)+4H2O(g)£¬”÷H<0£¬ŌŚŗćĪĀŗćČŻŹ±»Ų“šŅŌĻĀĪŹĢā£ŗ
Na2S(s)+4H2O(g)£¬”÷H<0£¬ŌŚŗćĪĀŗćČŻŹ±»Ų“šŅŌĻĀĪŹĢā£ŗ
(1)ĻņČŻĘ÷ÖŠ·Ö±š¼ÓČėŅŌĻĀĪļÖŹ£¬ÅŠ¶Ļ¶ŌĘ½ŗāÓŠĪŽÓ°Ļģ£¬ÓŠÓ°ĻģµÄÓ¦ĢīŠ“³öĘ½ŗāŅĘ¶ÆµÄ·½Ļņ”£
¢Ł¼ÓČėNa2SO4_________________”£¢Ś¼ÓČė½¹Ģæ________________”£
(2)Čō³õŹ±¼ÓČėµÄNa2SO4ŹĒ1£®42g£¬Ę½ŗāŹ±ČŻĘ÷ÖŠ¹ĢĢåĪļÖŹÖŹĮæŹĒ1£®10g£¬Na2SO4µÄ×Ŗ»ÆĀŹŹĒ_______________”£
(3)Čō½«ČŻĘ÷ĪĀ¶ČÉżøß20”ę£¬H2ŌŚ»ģŗĻĘųĢåÖŠŗ¬Įæ±ä»ÆÓĆĻĀĶ¼ÖŠ_____ Ķ¼±ķŹ¾×īŗĻŹŹ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com