
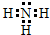 ��
������ 48.20g�����ʵ����ʵ�����$\frac{48.2g}{482g/mol}$=0.1mol�����100mL��Һ�����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol������������ˮ������Һ���ػ�ɫ������Fe3+��ȡ������Һ50mL����0.05mol�����У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g������NH4+��������0.85g�ǰ��������ʵ�����$\frac{0.85g}{17g/mol}$=0.05mol�����Ժ���笠�������0.05mol����1mol�����к���笠�����1mol�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壬��ΪFe2O3�����ʵ�����$\frac{4.00g}{160g/mol}$=0.025mol��������������0.05mol������1mol�����к���������1mol��0.05mol�������м���������BaCl2��Һ����������������İ�ɫ���������ᱵ 23.30g����0.1mol��1mol���������Ժ�����������ӵ����ʵ�����2mol��������ʵ�Ħ���������õ������ʵķ���ʽΪ��NH4Fe��SO4��2•12H2O��Ȼ����Ԫ�ػ�����֪ʶ�����
��� �⣺48.20g�����ʵ����ʵ�����$\frac{48.2g}{482g/mol}$=0.1mol�����100mL��Һ�����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol������������ˮ������Һ���ػ�ɫ������Fe3+��ȡ������Һ50mL����0.05mol�����У�����������0.1mol/L NaOH��Һ�����ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g������NH4+��������0.85g�ǰ��������ʵ�����$\frac{0.85g}{17g/mol}$=0.05mol�����Ժ���笠�������0.05mol����1mol�����к���笠�����1mol�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壬��ΪFe2O3�����ʵ�����$\frac{4.00g}{160g/mol}$=0.025mol��������������0.05mol������1mol�����к���������1mol��0.05mol�������м���������BaCl2��Һ����������������İ�ɫ���������ᱵ23.30g����0.1mol��1mol���������Ժ�����������ӵ����ʵ�����2mol��������ʵ�Ħ���������õ������ʵķ���ʽΪNH4Fe��SO4��2•12H2O��
��1������Ϊ�����������ĵ���ʽΪ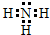 ���ʴ�Ϊ��
���ʴ�Ϊ��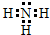 ��
��
��2�����ʵĻ�ѧʽΪNH4Fe��SO4��2•12H2O�������������ӷ�ˮ�ļ���Լ��������ʵȣ��ʴ�Ϊ��NH4Fe��SO4��2•12H2O�����ʣ�
��3��NH4Fe��SO4��2•12H2O��Һ����μ�������������Һ������Ԫ����ȫ����ʱ�Ļ�ѧ��Ӧ����ʽΪNH4Fe��SO4��2+3Ba��OH��2=��NH4��2SO4+3BaSO4��+2Fe��OH��3�����ʴ�Ϊ��NH4Fe��SO4��2+3Ba��OH��2=��NH4��2SO4+3BaSO4��+2Fe��OH��3����
��4�������Ӿ��������ԣ��ܽ�����������������Ӧ�ķ���ʽΪ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Һ���ػ�ɫ��Ϊdz��ɫ��
�ʴ�Ϊ����Һ���ػ�ɫ��Ϊdz��ɫ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+��
��5��ʵ�鷽����֤�������н��������ӵķ���Ϊȡ�����������Թ��У���ˮ����ܽ⣬�μ����軯����Һ������Һ��Ѫ��ɫ������֤����������һ������Fe3+��
�ʴ�Ϊ��ȡ�����������Թ��У���ˮ����ܽ⣬�μ����軯����Һ������Һ��Ѫ��ɫ������֤����������һ������Fe3+��
���� ���⿼��������ƶϣ�Ϊ��Ƶ���㣬���ճ�����������жϼ������ķ�Ӧ��Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע�ⳣ�����ӵļ��鷽����Ӧ�ã���Ŀ�ѶȲ���


 ��ѧ����ϵ�д�
��ѧ����ϵ�д� �ο�������ϵ�д�
�ο�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2H��3H��Ϊͬλ�� | B�� | ���ʯ��ʯī��Ϊͬ�������� | ||
| C�� | 3O2$\frac{\underline{\;�ŵ�\;}}{\;}$2O3�������仯 | D�� | �Ҵ�������ѻ�Ϊͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 25��ʱ����100mL��0.1mol•L-1��NH4HSO4��Һ�еμ�0.1mol•L-1��NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ����μӹ����������������������˵��������ǣ�������
25��ʱ����100mL��0.1mol•L-1��NH4HSO4��Һ�еμ�0.1mol•L-1��NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ����μӹ����������������������˵��������ǣ�������| A�� | δ�μ�NaOH��Һʱ��Һ��pHС����ͬ������0.1mol•L-1��NaHSO4��Һ��pH | |
| B�� | pHΪ7ʱ�����Һ��ˮ�ĵ���̶���� | |
| C�� | ��V��NaOH��=30mLʱ��c��NH3•H2O��+c��Na+����2c��SO42-�� | |
| D�� | �μ�NaOH��Һ�������30mL��40mL�Ĺ����У�$\frac{c��N{{H}_{4}}^{+}��}{c��{H}^{+}��}$��ֵ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
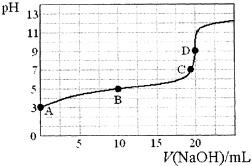 ��֪��H3PO2��һԪ��ǿ�ᣮ25�棬��20mL��0.1mol•L-1��H3PO2��Һ�еμ�0.1mol•L-1��NaOH��Һ���ζ���������Һ�¶ȱ��ֲ��䣩���ζ�������ͼ��������˵������ȷ���ǣ�������
��֪��H3PO2��һԪ��ǿ�ᣮ25�棬��20mL��0.1mol•L-1��H3PO2��Һ�еμ�0.1mol•L-1��NaOH��Һ���ζ���������Һ�¶ȱ��ֲ��䣩���ζ�������ͼ��������˵������ȷ���ǣ�������| A�� | H3PO2�ĵ��뷽��ʽΪ��H3PO2?H2PO2-+H+ | |
| B�� | �����£�Ka��H3PO2����10-5 | |
| C�� | �õζ�ʵ���У��÷�̪��ָʾ�����ü�����ָʾ�������С | |
| D�� | B����Һ�д��ڹ�ϵ��c��H+��+c��H3PO2��=c��OH-��+c��H2PO2-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
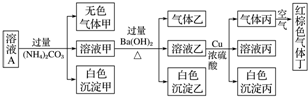
| A�� | ����Һ��һ����NO3-��Al3+��SO42-��Cl-�������� | |
| B�� | ʵ���������Cu 1.92g������������ڱ�״̬�����Ϊ448mL | |
| C�� | ������һ����BaCO3��������BaSO4 | |
| D�� | Ϊȷ��ԭ��Һ���Ƿ���Na+��K+����ͨ����ɫ��Ӧ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������������ЧӦ�����⻯ѧ���������γɶ��뵪���������� | |
| B�� | Һ������ʱҪ���մ������ȣ������������ | |
| C�� | ��ϸ��������ͭ��ʯ����ֱ��ת��Ϊ����ͭ��������̽���������ͭ | |
| D�� | ���۱�ը�¼��в���ȼ�յ�һ��Σ��Ʒ���ױ�������������TDI��������Σ��Ʒ���ڷ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����lmolO2��Mg��ȫ��Ӧ���4mol���ӣ����Ƴ�lmo1 O2������������ȫ��Ӧ�����4mol���� | |
| B�� | ��ͬpHֵ��H3PO4��H2SO4��Һ��ϡ����ͬ�����ٷֱ����pHֵ����H3PO4��Һ��pHС��H2SO4��Һ�����Ƴ�Ԫ�صķǽ�����S��P | |
| C�� | ����Cl2+2KI=2KCl+I2��Ӧ�У������ԣ�Cl2��I2�����Ƴ�SiO2+2C $\frac{\underline{\;����\;}}{\;}$Si+2CO����Ӧ�У������ԣ�C��Si | |
| D�� | ��3%H2O2��Һ�м�0.1gMnO2��ĩ�ȼ�2��1mol•L-1FeCl3��Һ��Ӧ���ң����ݴ�ʵ����Ƴ�MnO2�Ĵ�Ч��һ����FeCl3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ԭ������ | �����Ų�ʽ | �����ڱ��е�λ�� | �ǽ������Ƿǽ��� | ����������ˮ���P����� | ��̬�⻯��Ļ�ѧʽ |
| 15 | |||||
| 1s22s22p63s23p4 | |||||
| �ڶ�����VA�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com