
 £Ø1£©ŹµŃéŹŅÓĆČēĶ¼ĖłŹ¾×°ÖĆÖʱøÉŁĮæŅŅĖįŅŅõ„£®
£Ø1£©ŹµŃéŹŅÓĆČēĶ¼ĖłŹ¾×°ÖĆÖʱøÉŁĮæŅŅĖįŅŅõ„£®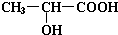 £®ŹŌ»Ų“š£ŗ
£®ŹŌ»Ų“š£ŗ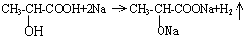 £»
£»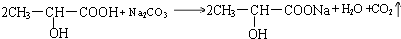 £®
£® ·ÖĪö £Ø1£©ŹµŃéŹŅÓĆŅŅ“¼”¢ŅŅĖįŌŚÅØĮņĖį×÷ÓĆĻĀ£¬¼ÓČČ·¢Éśõ„»Æ·“Ӧɜ³ÉŅŅĖįŅŅõ„£¬ŅŅĖįŅŅõ„²»ČÜÓŚ±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬ŅŅĖįæÉÓėĢ¼ĖįÄĘČÜŅŗ·“Ó¦£¬ŹŌ¹Ü¢ņÖŠŹ¢ÓŠ±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬×¢Ņā·ĄÖ¹µ¹Īü£»
£Ø2£©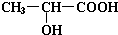 ŗ¬ÓŠōČ»ł£¬¾ßÓŠĖįŠŌ£¬æÉ·¢ÉśÖŠŗĶ”¢õ„»Æ·“Ó¦£¬ŗ¬ÓŠōĒ»ł£¬æÉ·¢ÉśČ”“ś”¢ĻūČ„ŗĶŃõ»Æ·“Ó¦£¬ŅŌ“Ė½ā“šøĆĢā£®
ŗ¬ÓŠōČ»ł£¬¾ßÓŠĖįŠŌ£¬æÉ·¢ÉśÖŠŗĶ”¢õ„»Æ·“Ó¦£¬ŗ¬ÓŠōĒ»ł£¬æÉ·¢ÉśČ”“ś”¢ĻūČ„ŗĶŃõ»Æ·“Ó¦£¬ŅŌ“Ė½ā“šøĆĢā£®
½ā“š ½ā£ŗ£Ø1£©¢Ł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖCH3CH2OH+CH3COOH$?_{”÷}^{ÅØH_{2}SO_{4}}$CH3COOCH2CH3+H2O£¬
¹Ź“š°øĪŖ£ŗCH3CH2OH+CH3COOH$?_{”÷}^{ÅØH_{2}SO_{4}}$CH3COOCH2CH3+H2O£»
¢ŚŹµŃéŹŅÓƱ„ŗĶĢ¼ĖįÄĘČÜŅŗĪüŹÕ£¬ŅņŅŅĖįŅŅõ„²»ČÜÓŚ±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬ĒŅ±„ŗĶĢ¼ĖįÄĘČÜŅŗæÉĘšµ½³żČ„ŅŅĖįŗĶŅŅ“¼µÄ×÷ÓĆ£¬¹Ź“š°øĪŖ£ŗ±„ŗĶNa2CO3ČÜŅŗ£»
¢ŪŅŅĖįŅŅõ„²»ČÜÓŚ±„ŗĶĢ¼ĖįÄĘČÜŅŗ£¬æÉÓĆ·ÖŅŗµÄ·½·Ø·ÖĄė£¬¹Ź“š°øĪŖ£ŗ·ÖŅŗ£»
£Ø2£©¢ŁÓɽį¹¹¼ņŹ½æÉÖŖČéĖįŗ¬ÓŠōĒ»ł”¢ōČ»ł£¬¹Ź“š°øĪŖ£ŗōĒ»ł£»ōČ»ł£»
¢ŚČéĖįÖŠŗ¬ÓŠ-OHŗĶ-COOH£¬¶¼ÄÜÓėNa·“Ӧɜ³ÉĒāĘų£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ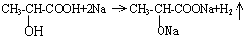 £¬
£¬
¹Ź“š°øĪŖ£ŗ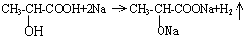 £»
£»
¢ŪČéĖįÖŠµÄ-COOH¾ßÓŠĖįŠŌ£¬ÄÜÓėĢ¼ĖįÄĘ·“Ӧɜ³É¶žŃõ»ÆĢ¼ĘųĢ壬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ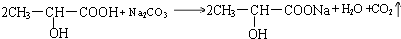 £¬
£¬
¹Ź“š°øĪŖ£ŗ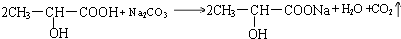 £®
£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄÖʱøŹµŃ飬ŅŌ¼°ÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ£¬ĪŖøßĘµæ¼µć£¬²ąÖŲ·ÖĪöÓėŹµŃéÄÜĮ¦µÄ漲飬°ŃĪÕÖʱøŹµŃé²Ł×÷”¢»ģŗĻĪļ·ÖĄėĢį“攢ӊ»śĪļµÄŠŌÖŹĪŖ½ā“šµÄ¹Ų¼ü£¬×¢Ņā°ŃĪÕÓŠ»śĪļ¹ŁÄÜĶŵĊŌÖŹ£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 23 g Na±äĪŖNa+Ź±µĆµ½µÄµē×ÓŹżĪŖNA | |
| B£® | 18 gĖ®Ėłŗ¬µÄµē×ÓŹżĪŖNA | |
| C£® | 16 g O2Óė16 g O3Ėłŗ¬µÄŌ×ÓŹż¾łŹĒNA | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬46 gµÄNO2ŗĶN2O4»ģŗĻĘųĢåŗ¬ÓŠµÄ·Ö×ÓŹżĪŖ3NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ż | B£® | ¢Ł¢Ś¢Ž | C£® | ¢Ś¢Ū¢Ž | D£® | ³ż¢Ü¢ß |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĒāŃõ»ÆĢśÓėŃĪĖį·“Ó¦OH-+H+ØTH2O | |
| B£® | ÓĆĻ”ĮņĖįĒå³żĢśŠāFe2O3+6H+ØT2Fe3++3H2O | |
| C£® | Ź³ŃĪĖ®ÖŠµĪ¼ÓĻõĖįŅųČÜŅŗCl-+Ag+ØTAgCl”ż | |
| D£® | ĒāŃõ»Æ±µøśĮņĖįĶČÜŅŗµÄ·“Ó¦Ba2++2OH-+Cu2++SO42-ØTBaSO4”ż+Cu£ØOH£©2”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | $\frac{1}{\sqrt{a}}$ | B£® | $\sqrt{a}$ | C£® | $\frac{1}{2}$a | D£® | $\frac{1}{\frac{1}{2}a}$ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
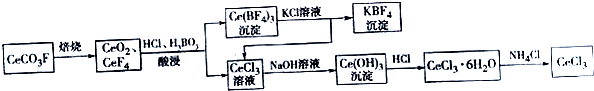
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
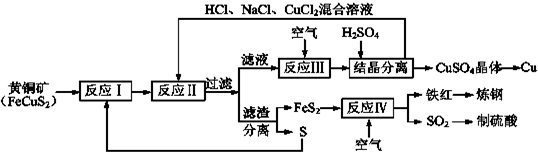
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com