
����ͼװ����ʾ��C��D��E��F��X��Y���Ƕ��Ե缫���ס�������Һ�������Ũ�ȶ���ͬ(����ͨ��ǰ����Һ�������)��A��BΪ���ֱ����Դ����������ֱ����Դ��ͨ��F�������ʺ�ɫ��
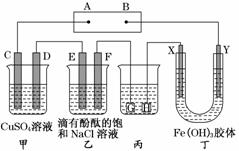
��ش�
(1)B���ǵ�Դ��______________��һ��ʱ�������Һ��ɫ________������X����������ɫ��dz��Y����������ɫ��������_____________________________��
�ڵ糡��������Y���ƶ���
(2)���ס���װ���е�C��D��E��F�缫��ֻ��һ�ֵ�������ʱ����Ӧ���ʵ����ʵ���֮��Ϊ________��
(3)���ñ�װ�ø�ͭ����������HӦ��______________(��Ʋ�������Ƽ���)�����Һ��________��Һ����������Һ��pH��13ʱ (��ʱ����Һ���Ϊ500 mL)�����жƼ���������������Ϊ________��������Һ��pH________(������С�����䡱)��
(4)����C�缫��Ϊ��������װ�ö����䣬����з������ܷ�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
������(3)��ͭ�����������ݵ��ԭ����ͭ�����������������������Һ�ǿ��������Ρ���������Һ��pH��13ʱ��������n(OH��)��0.1 mol/L��0.5 L��0.05 mol�������缫ת�Ƶ���0.05 mol�����Ա���������0.05 mol����װ�������ڵ�����H����������Һ��������ǿ��pH��С��
�𰸣�(1)��������dz���������������������
(2)1��2��2��2
(3)�Ƽ���AgNO3(��������)��5.4 g����С
(4)Fe��Cu2��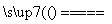 Cu��Fe2��
Cu��Fe2��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�ﵽԤ�ڵ�ʵ��Ŀ�ģ����з������е���
A�����ܽ⡢���˵ķ����ᴿ�����������ᱵ��̼�ᱵ
B��Ϊ��С�к͵ζ�����ƿ����ϴ������ɺ����ʹ��
C������ˮ�����顢��ϩ�����Ȼ�̼���Ҵ�������ˮ��Һ5����ɫҺ��
D���������������������м���NaOH��Һ��������Һ���ᴿ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪4NH3+5O2��4NO+6H2O ������Ӧ���ʷֱ���V(NH3)�� V(O2)��V(NO)��V(H2O) ��ʾ������ȷ�Ĺ�ϵ�ǣ� ��
A.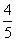 V(NH3)=V(O2) B.
V(NH3)=V(O2) B.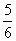 V(O2)=V(H2O) C.
V(O2)=V(H2O) C.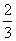 V(NH3)=V(H2O) D.
V(NH3)=V(H2O) D.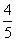 V(O2)=V(NO)
V(O2)=V(NO)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����ܸ�̼������Һ��Ӧ���ɱ����ơ���������ת����




 COOH COONa COONa COONa
COOH COONa COONa COONa




 HO��
HO��




CH2OH CH2OH CH2OH CH2ONa
��������ת����ϵ�ش��������⣺
��1��a.�Ȼ� b.���ǻ� c.���ǻ��ṩ���ӵ�������(����)_____________________��
��2���١��ݱ�����õ��Լ��ֱ��ǣ���_____________����_____________��
��_______________����________________ ����________________��
��3��д��������̼������Һ��Ӧ�����ӷ���ʽ
_________________________________________________��
��4��д���Ҵ�����ˮ��Ӧ�Ļ�ѧ����ʽ
________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ǧ���ص��AgNO3��Na2SO3����Һ��a��b��c��d�缫���Ͼ�Ϊʯī����֪Ǧ���ص��ܷ�ӦΪ��Pb(s)��PbO2(s)��2H2SO4(aq) 2PbSO4(s)��2H2O(l)��ͨ��ʱa�缫�������ӣ�����˵����ȷ����(����)
2PbSO4(s)��2H2O(l)��ͨ��ʱa�缫�������ӣ�����˵����ȷ����(����)
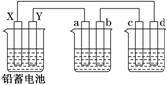
A����·��ͨ��1 mol����ʱ�� Y�缫��������48 g
B���ŵ�ʱǦ���ظ����ĵ缫��ӦʽΪ��
PbO2(s)��4H��(aq)��SO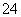 (aq)��2e��===PbSO4(s)��2H2O(l)
(aq)��2e��===PbSO4(s)��2H2O(l)
C��c��d�缫������������ʵ���֮��Ϊ1��2
D��X������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦaM(g)��bN(g)cP(g)��dQ(g)�ﵽƽ��ʱ��M���������y(M)�뷴Ӧ�����Ĺ�ϵ��ͼ��ʾ������z��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȡ�����˵����ȷ����(����)
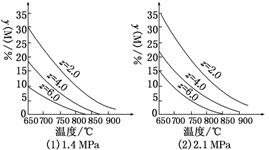
A��ͬ��ͬѹͬzʱ�����������ƽ��ʱQ�������������
B��ͬѹͬzʱ�������¶ȣ�ƽ��ʱQ�������������
C��ͬ��ͬzʱ������ѹǿ��ƽ��ʱQ�������������
D��ͬ��ͬѹʱ������z��ƽ��ʱQ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���淴ӦN2��3H22NH3�ǹ�ҵ�Ϻϳɰ�����Ҫ��Ӧ��
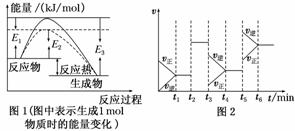
(1)����ͼ1��д���ϳɰ����Ȼ�ѧ����ʽ
________________________________________________________________________
________________________________________________________________________
(������E1��E2��E3��ʾ)��
(2)ͼ1�����߲�����ͨ���ı仯ѧ��Ӧ�е�________�������������ĸı���ͼ2����һʱ�������ĸı���ͬ________(�á�t1����t6����ʾ)��
(3)ͼ2��t3ʱ�̸ı��������________________��t5ʱ�̸ı��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0��1.01��105Pa�£���H2��O2��CH4��������������ȷ���ǣ���
| A�� | ���ܶ�֮�ȵ������ʵ���֮�� | |
| B�� | ���ܶ�֮�ȵ���Ħ������֮�� | |
| C�� | ���������������壬�����֮�ȵ�����Է��������ĵ����� | |
| D�� | ��������������壬�����ʵ���֮�ȵ�����Է�������֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ʯSiC�Ļ�ѧ����ʽ�ǣ�SiO2+3C SiC+2CO������Ҫ��ش��������⣺
SiC+2CO������Ҫ��ش��������⣺
��1��ָ������������ԭ�����������
��2���������ת�Ƶķ������Ŀ��
��3����30gSiO2��24gC���Ƶö��ٿ�SiC��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com