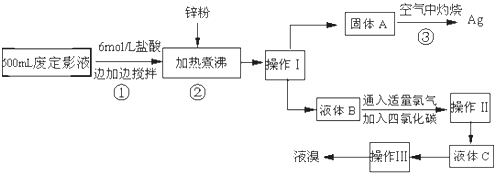
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,��ѧ����ʽ���йؼ���,������ԭ��Ӧ����ʽ����ƽ,�ȡ��塢�⼰�仯������ۺ�Ӧ��
ר�⣺������,ʵ�������,Ԫ�ؼ��仯����
��������1��ˮ���ܽ������Ŀ�����Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mg CaO����֮�൱�����ʣ���7.1mg MgO������ˮ�е�Ca
2+��Mg
2+���������CaO����������õ���
��2�����ñ����ӳ�ȥ��������ӣ������ü��ȥCa
2+��Mg
2+��HCO
3-��
��3������Ļ�������������������������������������������������������ˮ���������ʣ�
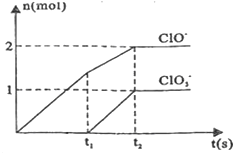
��4����ͼ��֪n��ClO
-��=2mol��n��ClO
3-��=1mol���ɵ�ʧ�����غ��֪n��Cl
-��=5n��ClO
3-��+n��ClO
-��=5��1+1��2=7mol����Ϊǡ����ȫ��Ӧ����NaCl��NaClO��NaClO
3��������غ㣬��֪n��NaOH��=n��Cl
-��+n��ClO
3-��+n��ClO
-��=7+1+2=10mol������V=
=
=2.5L��
��5����CN
-������̼�뵪ԭ��֮��Ϊ1��1����̼��ת��ΪCNO
-��N
2�Լ�CO
32-������ΪCNO
-����̼��֮��Ϊ1��1������N
2��CO
32-����֮��Ϊ1��2��
��6���裨CN��
2�Ļ�ѧ������±�أ�X
2�������ƣ�����CN��
2��ˮ��Ӧ������HCN��HCNO����CN��
2�������Ա�Br
2������I
2ǿ������±�ص��ʵ����ʷ����ж�ѡ�
���
�⣺��1����Ȼˮ���ܽ��������Ҫ��������������̼����������ij��Ȼˮ��c��Ca
2+��=1.2��10
-3mol?L
-1��c��Mg
2+��=6��10
-4mol?L
-1��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO����1Lˮ�и��������ʵ���=1.2��10
-3mol���൱��CaO����=1.2��10
-3mol��56g/mol=67.2mg��1Lˮ��þ�������ʵ���=6��10
-4mol���൱������þ����6��10
-4mol��40g/mol=24mg������ˮ��Ӳ��=
+
=10��
�ʴ�Ϊ��������������̼����������10�㣻
��2�������ӳ�ȥ��������ӣ������ü��ȥCa
2+��Mg
2+��HCO
3-���ҹ����ı������ܱ�̼������ӳ�ȥ������a�������Լ�������˳��ΪBaCl
2��CaO��
�ʴ�Ϊ��BaCl
2��CaO��
��3������Ļ������������������������������������������ȣ�������������ˮ���������ʣ���ˮԭ�������ӷ���ʽ�ɱ�ʾΪAl
3++3H
2O?Al��OH��
3+3H
+��Fe
3++3H
2O?Fe��OH��
3+3H
+��Fe
2++2H
2O?Fe��OH��
2+2H
+��
�ʴ�Ϊ����������������������������������
��4����ͼ��֪n��ClO
-��=2mol��n��ClO
3-��=1mol���ɵ�ʧ�����غ��֪n��Cl
-��=5n��ClO
3-��+n��ClO
-��=5��1+1��2=7mol����Ϊǡ����ȫ��Ӧ����NaCl��NaClO��NaClO
3��������غ㣬��֪n��NaOH��=n��Cl
-��+n��ClO
3-��+n��ClO
-��=7+1+2=10mol������V=
=
=2.5L���ʴ�Ϊ��2.5��
��5����CN
-������̼�뵪ԭ��֮��Ϊ1��1����̼��ת��ΪCNO
-��N
2�Լ�CO
32-������ΪCNO
-����̼��֮��Ϊ1��1������N
2��CO
32-����֮��Ϊ1��2������e��f=1��2����ѡB��
��6���裨CN��
2�Ļ�ѧ������±�أ�X
2�������ƣ�����CN��
2��ˮ��Ӧ������HCN��HCNO����CN��
2�������Ա�Br
2�����ȱ�I
2ǿ��
A����CN��
2��NaOH��Һ��Ӧ������������������������Һ�ķ�Ӧ�����Է��õ����ӷ���ʽΪ����CN��
2+2OH
-�TCN
-+CNO
-+H
2O����A��ȷ��
B��MnO
2��HCN��Ӧ�������ƶ������̺�Ũ����ķ�Ӧ�����Է��õĻ�ѧ����ʽΪ��MnO
2+4HCN
Mn��CN��
2+��CN��
2��+2H
2O����B��ȷ��
C����KCN��Һ�м����ˮ�����������û���Ӧ������CN��
2�������Ա�I
2ǿ�����ܷ�����ӦI
2+2KCN�T��2KI��+��CN��
2����C����
D����NaBr��KCN�����Һ��ͨ������Cl
2����CN��
2�������Ա�Br
2�������������ӻ�ԭ��С��CN
-���ӣ��ȷ�����ӦCl
2+2CN
-�T2Cl
-+��CN��
2����D����
�ʴ�Ϊ��CD��
���������⿼����ۺϣ��漰���ʺ����IJⶨ������ˮ���Ӧ�á���ˮ����������ԭ����ˮ��Ӳ�ȼ��㼰������ԭ��Ӧ����ȣ����������Ϣ��֪ʶǨ��Ӧ��Ϊ���Ĺؼ������ط��������Ŀ��飬��Ŀ�Ѷ��еȣ�



 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
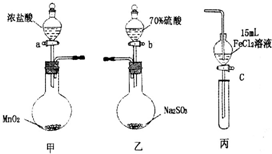 Ϊ��֤������C12��Fe3+��SO2ijС������ͼ��ʾװ�ý���ʵ�飨�г������ͼ��м���װ�����ԣ��������Ѽ��飩��
Ϊ��֤������C12��Fe3+��SO2ijС������ͼ��ʾװ�ý���ʵ�飨�г������ͼ��м���װ�����ԣ��������Ѽ��飩��