
»ĘĢśæó£ØÖ÷ŅŖ³É·ÖĪŖFeS2£©ŹĒĪŅ¹ś“󶹏żĮņĖį³§ÖĘČ”ĮņĖįµÄÖ÷ŅŖŌĮĻ”£Ä³»ÆѧѧĻ°Š”×é¶Ōij»ĘĢśæóŹÆ½ųŠŠČēĻĀŹµŃéĢ½¾æ”£
[ŹµŃéŅ»]²ā¶ØĮņŌŖĖŲµÄŗ¬Į攣
¢ń”¢½«m1 gøĆ»ĘĢśæóѳʷ·ÅČėČēĻĀĶ¼ĖłŹ¾×°ÖĆ£Ø¼Š³ÖŗĶ¼ÓČČ×°ÖĆŹ”ĀŌ£©µÄŹÆÓ¢¹ÜÖŠ£¬“Óa“¦²»¶ĻµŲ»ŗ»ŗĶØČėæÕĘų£¬øßĪĀ×ĘÉÕŹÆÓ¢¹ÜÖŠµÄ»ĘĢśæóѳʷÖĮ·“Ó¦ĶźČ«”£ŹÆÓ¢¹ÜÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ4FeS2+11O2 2Fe2O3+8SO2
2Fe2O3+8SO2
¢ņ”¢·“Ó¦½įŹųŗ󣬽«ŅŅĘæÖŠµÄČÜŅŗ½ųŠŠČēĻĀ“¦Ąķ£ŗ
|

£Ø1£©ĒāŃõ»ÆÄĘ£Ø»ņĒāŃõ»Æ¼Ų£©£Ø2·Ö£© SO2+2OH”Ŗ=SO32”Ŗ+H2O£»£Ø2SO32”Ŗ+O2=2SO42”ŖĪ“Š“²»æŪ·Ö£©£Ø2·Ö£©
£Ø2£©SO32”Ŗ+H2O2=SO42”Ŗ+H2O £Ø2·Ö£©
£Ø3£©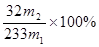 £Ø2·Ö£©
£Ø2·Ö£©
£Ø4£©250mlČŻĮæĘæ£Ø2·Ö£©
£Ø5£©×īŗóŅ»µĪøßĆĢĖį¼ŲČÜŅŗµĪČėŹ±£¬ČÜŅŗŃÕÉ«Ķ»±äĪŖ×ĻÉ«£¬ĒŅŌŚ30sÄŚ²»±äÉ«”££Ø2·Ö£©
£Ø6£©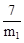 »ņ
»ņ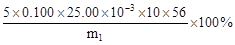 »ņĘäĖüŗĻĄķ“š°ø£Ø2·Ö£©
»ņĘäĖüŗĻĄķ“š°ø£Ø2·Ö£©
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ĪŖ·ĄÖ¹SO2½ųČėµ½¼××°ÖĆÖŠ£¬æÉÓĆĒāŃõ»ÆÄĘ£Ø»ņĒāŃõ»Æ¼Ų£©ĪüŹÕ£¬ŅŅĘæÖŠµÄĒāŃõ»ÆÄĘĪüŹÕÓ²ÖŹ²£Į§¹ÜÖŠ²śÉśµÄ¶žŃõ»ÆĮņ£¬·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗSO2+2OH”Ŗ=SO32”Ŗ+H2O£¬ÓÉӌ׶ŠĪĘæÖŠŅ²“ęŌŚ×ÅĪ“ĶźČ«·“Ó¦µÄŃõĘų£¬¹ŹŅ²·¢Éś2SO32”Ŗ+O2=2SO42”Ŗ£Ø2£©IIÖŠ£¬ŅŅĘæ¼ÓČėH2O2ČÜŅŗŹ±·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖSO32”Ŗ+H2O2=SO42”Ŗ+H2O£»£Ø3£©ŅŅĘæÖŠµÄČÜŅŗ²śÉśSO42”Ŗ£¬SO42”ŖÓė¼ÓČėµÄĀČ»Æ±µÖŠµÄBa2+³ĮµķĻą½įŗĻ·¢Éś³Įµķ·“Ó¦£ŗSO42”Ŗ+Ba2+=BaSO4”ż£¬¼“¹ĢĢåm2gĪŖBaSO4£¬½įŗĻĢāŅāĮŠ¹ŲĻµŹ½ČēĻĀ£ŗ
FeS2”Ŗ2SO42”Ŗ”Ŗ2BaSO4
1mol 2mol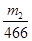
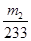
ÓÉ“ĖæÉÖŖ£¬ĮņŌŖĖŲŌŚFeS2ÖŠµÄĪļÖŹµÄĮæĪŖ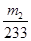 £¬ŌņĘäÖŹĮæĪŖ£ŗ
£¬ŌņĘäÖŹĮæĪŖ£ŗ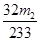 £¬ĖłŅŌøĆ»ĘĢśæóÖŠĮņŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ
£¬ĖłŅŌøĆ»ĘĢśæóÖŠĮņŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ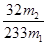 ”Į©£»£Ø4£©Ó¦ĪŖ250mlČŻĮæĘ棻£Ø5£©ŅņĪŖĪ“¼ÓČėKMnO4ČÜŅŗÖ®Ē°£¬ČÜŅŗÖŠŗ¬ÓŠFe2+£¬ČÜŅŗĪŖĒ³ĀĢÉ«£¬
”Į©£»£Ø4£©Ó¦ĪŖ250mlČŻĮæĘ棻£Ø5£©ŅņĪŖĪ“¼ÓČėKMnO4ČÜŅŗÖ®Ē°£¬ČÜŅŗÖŠŗ¬ÓŠFe2+£¬ČÜŅŗĪŖĒ³ĀĢÉ«£¬
µ±¼ÓČė×īŗóŅ»µĪøßĆĢĖį¼ŲČÜŅŗµĪČėŹ±£¬ČÜŅŗŃÕÉ«Ķ»±äĪŖ×ĻÉ«£¬ĒŅŌŚ30sÄŚ²»±äÉ«£¬ŌņÖ¤Ć÷µ½“ļµĪ¶ØÖÕµć”£
£Ø6£©·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ5Fe2++8H++MnO4-=Mn2++5Fe3++4H2O,Č»ŗóøł¾Ż5Fe2+”ŖMnO4-ĮŠ¹ŲĻµŹ½æÉĒóµĆ
øĆ»ĘĢśæóѳʷĢśŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ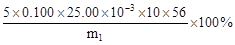 »ņ
»ņ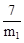
æ¼µć£ŗæ¼²éĪļÖŹµÄ·ÖĄėÓėĢį“棬»ÆѧŹµŃ黳±¾²Ł×÷”¢Ńõ»¹Ō·“Ó¦”¢Ąė×Ó·½³ĢŹ½µÄŹéŠ“”¢Ńõ»Æ»¹Ō·“Ó¦µĪ¶ØŌĄķ
Óė¼ĘĖć”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÓĆ“óĄķŹÆµČŌĮĻÖĘČ”°²Č«ĪŽ¶¾µÄɱ¾ś¼Į¹żŃõ»ÆøĘ”£“óĄķŹÆµÄÖ÷ŅŖŌÓÖŹŹĒŃõ»ÆĢś£¬ŅŌĻĀŹĒĢį“æ“óĄķŹÆµÄŹµŃé²½Öč£ŗ

£Ø1£©ŅŃÖŖĀĖŅŗBµÄÖ÷ŅŖ³É·ŻŹĒĻõĖįļ§£¬ŌņĖįXĪŖ_______£ØĢīĆū³Ę£¬ĻĀĶ¬£©£¬AĪļÖŹĪŖ_______”£
£Ø2£© ¼ģŃé²Ł×÷IIŗóČÜŅŗÖŠŹĒ·ń»¹ŗ¬ĢśĄė×ӵďŌ¼ĮŹĒ £ØĢī»ÆѧŹ½£©£¬Čē¹ūÓŠŌņ¹Ū²ģµ½µÄĻÖĻóŹĒ____________”£
¼ģŃé²Ł×÷IIŗóČÜŅŗÖŠŹĒ·ń»¹ŗ¬ĢśĄė×ӵďŌ¼ĮŹĒ £ØĢī»ÆѧŹ½£©£¬Čē¹ūÓŠŌņ¹Ū²ģµ½µÄĻÖĻóŹĒ____________”£
£Ø3£© Š“³ö¼ÓČėĢ¼Ėįļ§Ėł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
Š“³ö¼ÓČėĢ¼Ėįļ§Ėł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ ”£
£Ø4£© CaO2æÉ×÷¹©Ńõ¼Į£¬Š“³öCaO2ÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ____________”£
£Ø5£©CaO2ÖŠŅ»°ćŗ¬CaO£¬Ä³Ģ½¾æŠ”×é°“ĻĀĮŠ¹ż³Ģ²āĮæCaO2ŗ¬Įæ£ŗŹ×ĻČ³ĘČ”0.80gѳʷ£¬Č»ŗó½«ŃłĘ·ČÜÓŚ100mL 1.0mol/LµÄŃĪĖįÖŠ£¬ŹÕ¼Æµ½µÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ112mL£¬ŌņøĆѳʷ֊CaO2ŗ¬ĮæĪŖ___________”£
£Ø6£©ŅŖÅäÖĘ100mL 1.0mol/LµÄŃĪĖį£¬ŠčŅŖ12.5mol/LŃĪĖįµÄĢå»żĪŖ______mL£»ÅäÖĘøĆČÜŅŗŹ±£¬³żÓƵ½ĮæĶ²”¢ÉÕ±”¢½ŗĶ·µĪ¹ÜĶā£¬»¹ŠčŅŖµÄ²£Į§ŅĒĘ÷ŗĶÓĆĘ·ÓŠ______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(14·Ö)½üÄźĄ“ĪŅ¹śµÄŗ½ĢģŹĀŅµČ”µĆĮĖ¾Ž“óµÄ³É¾Ķ£¬ŌŚŗ½Ģģ·¢É䏱£¬ėĀ(N2H4)¼°ĘäŃÜÉśĪļ³£ÓĆ×÷»š¼żĶĘ½ų¼Į”£
¢ÅŅŗĢ¬ėĀ×÷»š¼żČ¼ĮĻŹ±£¬ÓėŅŗĢ¬N2O4»ģŗĻ·¢ÉśŃõ»Æ»¹Ō·“Ó¦£¬ŅŃÖŖĆæ1 gėĀ³ä·Ö·“Ó¦ŗóÉś³ÉĘųĢ¬Ė®·Å³öČČĮæĪŖa KJ£¬ŹŌŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ ”£
¢ĘŌŚŹµŃéŹŅÖŠ£¬ÓĆN2H4”¤H2OÓėNaOHæÅĮ£Ņ»ĘšÕōĮó£¬ŹÕ¼Æ114”«116 ”ęµÄĮó·Ö¼“ĪŖĪŽĖ®ėĀ”£
¢ŁŌŚÕōĮó¹ż³ĢÖŠ²»ŠčŅŖµÄŅĒĘ÷ŹĒ (ĢīŠņŗÅ×ÖÄø)”£
| A£®¾Ę¾«µĘ | B£®³¤Ö±²£Į§µ¼¹Ü | C£®×¶ŠĪĘæ | D£®ĄäÄż¹Ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖ²ā¶ØijĀĮĆ¾ŗĻ½šÖŠĆ¾µÄÖŹĮæ·ÖŹż£¬Ä³Š”×é¼Ę»®½«ĀĮĆ¾ŗĻ½šÓė×ćĮæĻ”ĮņĖįČÜŅŗ·“Ó¦£¬²ā¶ØÉś³ÉĘųĢåµÄĢå»ż”£ĢīŠ“ĻĀĮŠæÕ°×”£
£Ø1£©Ķ¬Ń§ĆĒŃ”ÓĆ¼××°ÖĆ½ųŠŠŹµŃé£ŗŹµŃéæŖŹ¼Ź±£¬ĻČ“ņæŖ·ÖŅŗĀ©¶·ÉĻæŚµÄ²£Į§Čū£¬ŌŁĒįĒį“ņæŖĘä»īČū£¬Ņ»»į¶łŗóĻ”ĮņĖį¾Ķ²»ÄÜĖ³ĄūµĪČė׶ŠĪĘ攣ĒėÄć°ļÖś·ÖĪöŌŅņŹĒ________________”£
£Ø2£©Ķ¬Ń§ĆĒ¾ĢÖĀŪČĻĪŖ¼××°ÖĆÓŠĮ½øö·½Ćę»įŅżĘš½Ļ“óĪó²ī£¬·Ö±šŹĒ__________ŗĶ__________”£
£Ø3£©ÓŚŹĒĖūĆĒÉč¼ĘĮĖŹµŃé×°ÖĆŅŅ”£ŅŅÖŠµ¼¹ÜaµÄ×÷ÓĆŹĒ__________”£ČōŹµŃéĒ°ŗóµĪ¶Ø¹ÜÖŠŅŗĆę¶ĮŹż·Ö±šĪŖV1 mL”¢V2 mL”£Ōņ²śÉśĒāĘųµÄĢå»żĪŖ______mL”£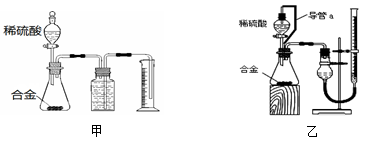
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖĢ½¾æijĢśĢ¼ŗĻ½šÓėÅØĮņĖįŌŚ¼ÓČČĢõ¼žĻĀµÄ·“Ó¦µÄ²æ·Ö²śĪļ²¢²ā¶ØĢśĢ¼ŗĻ½šÖŠĢśŌŖĖŲµÄÖŹĮæ·ÖŹż,ij»Æѧ»ī¶ÆŠ”×éÉč¼ĘĮĖČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ,²¢Ķź³ÉŅŌĻĀŹµŃéĢ½¾æ”£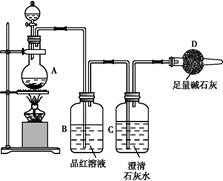
(1)ĶłŌ²µ×ÉÕĘæÖŠ¼ÓČėm gĢśĢ¼ŗĻ½š,²¢µĪČė¹żĮæÅØĮņĖį,Ī“µćČ¼¾Ę¾«µĘĒ°,A”¢B¾łĪŽĆ÷ĻŌĻÖĻó,ĘäŌŅņŹĒ:¢Ł³£ĪĀĻĀ,FeŌŚÅØĮņĖįÖŠ¶Ū»Æ;¢Ś”””””””””””””””””£
(2)·“Ó¦Ņ»¶ĪŹ±¼äŗó,“ÓAÖŠŅŻ³öĘųĢåµÄĖŁĀŹČŌČ»½Ļæģ,³żŅņ·“Ó¦ĪĀ¶Č½ĻøßĶā,»¹æÉÄܵÄŌŅņŹĒ”” ”£
(3)×°ÖĆBµÄ×÷ÓĆŹĒ”” ”£
(4)¼×Ķ¬Ń§¹Ū²ģµ½×°ÖĆCÖŠÓŠ°×É«³ĮµķÉś³É,ĖūµĆ³öĮĖŹ¹³ĪĒåŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢåŹĒ¶žŃõ»ÆĢ¼”£×°ÖĆAÖŠÄܲśÉś¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½ĪŖ”” ”£
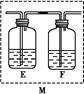
(5)ŅŅĶ¬Ń§ČĻĪŖ¼×Ķ¬Ń§µÄ½įĀŪŹĒ“ķĪóµÄ,ĖūČĻĪŖĪŖĮĖČ·ČĻ¶žŃõ»ÆĢ¼µÄ“ęŌŚ,ŠčŌŚ×°ÖĆB-CÖ®¼äĢķ¼Ó×°ÖĆM”£×°ÖĆE”¢FÖŠŹ¢·ÅµÄŹŌ¼Į·Ö±šŹĒ”””””””””””””””””””¢”””””””””””””””””””£ÖŲŠĀŹµŃéŗó¹Ū²ģµ½×°ÖĆFÖŠµÄĻÖĻóŹĒ”” ”””£
(6)ÓŠŠ©Ķ¬Ń§ČĻĪŖŗĻ½šÖŠĢśŌŖĖŲµÄÖŹĮæ·ÖŹżæÉÓĆKMnO4ČÜŅŗĄ“²ā¶Ø(5Fe2++Mn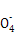 +8H+
+8H+ 5Fe3++Mn2++4H2O)ӣ
5Fe3++Mn2++4H2O)ӣ
²ā¶ØĢśŌŖĖŲÖŹĮæ·ÖŹżµÄŹµŃé²½ÖčČēĻĀ:
¢ń.ĶłÉÕĘæAÖŠ¼ÓČė¹żĮæµÄ»¹Ō¼ĮŹ¹ČÜŅŗÖŠµÄFe3+ĶźČ«×Ŗ»ÆĪŖFe2+,¹żĀĖ,µĆµ½ĀĖŅŗB;
¢ņ.½«ĀĖŅŗBĻ”ŹĶĪŖ250 mL;
¢ó.Č”Ļ”ŹĶŅŗ25.00 mL,ÓĆÅضČĪŖc mol”¤L-1µÄĖįŠŌKMnO4ČÜŅŗµĪ¶Ø,Čż“ĪµĪ¶ØŹµŃéĖłŠčKMnO4ČÜŅŗĢå»żµÄĘ½¾łÖµĪŖV mL”£
¢Ł²½Öč¢ņÖŠ,½«ĀĖŅŗBĻ”ŹĶĪŖ250 mLŠčŅŖÓƵ½µÄ²£Į§ŅĒĘ÷³żÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹ÜĶā,»¹±ŲŠėŅŖÓƵ½µÄŹĒ”””””””””£
¢Ś±ūĶ¬Ń§Éč¼ĘĮĖĻĀĮŠµĪ¶Ø·½Ź½(¼Š³ÖŅĒĘ÷Ź”ĀŌ),×īŗĻĄķµÄŹĒ””””””””(Ģī×ÖÄøŠņŗÅ)”£ 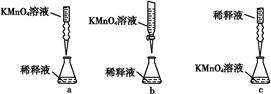
¢ŪµĪ¶Ø¹ż³ĢÖŠ””””””””(Ģī”°ŠčŅŖ”±»ņ”°²»ŠčŅŖ”±)¼ÓČėÖøŹ¾¼Į”£
¢ÜĢśĢ¼ŗĻ½šÖŠĢśŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ”” ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijĶ¬Ń§½ųŠŠĮņĖįĶ¾§Ģå½į¾§Ė®ŗ¬ĮæµÄ²ā¶ØŹµŃ锣Ķź³ÉĻĀĮŠĢīæÕ£ŗ
”¾ŹµŃé²½Öč”æ
£Ø1£©ÓĆ_______£ØĢīŅĒĘ÷Ćū³Ę£¬ĻĀĶ¬£©×¼Č·³ĘĮæ“ÉŪįŪöµÄÖŹĮ攣
£Ø2£©ŌŚ“ÉŪįŪöÖŠ¼ÓČėŌ¼2 gŃŠĻøµÄĮņĖįĶ¾§Ģ壬²¢³ĘĮ攣
£Ø3£©°ŃŹ¢ÓŠĮņĖįĶ¾§ĢåµÄ“ÉŪįŪö·ÅŌŚÄąČż½ĒÉĻĀżĀż¼ÓČČ£¬Ö±µ½Ą¶É«ĶźČ«±ä°×£¬Č»ŗó°ŃŪįŪöŅĘÖĮ____________ÖŠĄäČ“µ½ŹŅĪĀ£¬²¢³ĘĮ攣
£Ø4£©ÖŲø“(3)µÄŹµŃé½ųŠŠŗćÖŲ²Ł×÷£¬Ö±ÖĮĮ½“Ī³ĘĮæ½į¹ūĻą²ī²»³¬¹ż0.001 g”£
”¾Źż¾Ż¼ĒĀ¼Óė“¦Ąķ”æ
| | µŚŅ»“ĪŹµŃé | µŚ¶ž“ĪŹµŃé |
| ŪįŪöµÄÖŹĮæ£Øg£© | 29.563 | 30.064 |
| ŪįŪö£«ŹŌŃłµÄÖŹĮæ£Øg£© | 31.676 | 32.051 |
| ŗćÖŲŗó£¬ŪįŪö£«ĮņĖįĶµÄÖŹĮæ£Øg£© | 30.911 | 31.324 |
| xµÄÖµ | 5.05 | 5.13 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŹµŃéŹŅ³£ĄūÓĆ¼×Č©£ØHCHO£©·Ø²ā¶Ø(NH4)2SO4ѳʷ֊µŖµÄÖŹĮæ·ÖŹż£¬Ęä·“Ó¦ŌĄķĪŖ£ŗ4NH4£« £«6HCHO =3H£«£«6H2O£«(CH2)6N4H£« £ŪµĪ¶ØŹ±£¬1 mol (CH2)6N4H£«Óė l mol H£«Ļąµ±£¬Č»ŗóÓĆNaOH±ź×¼ČÜŅŗµĪ¶Ø·“Ӧɜ³ÉµÄĖį”£Ä³ŠĖȤŠ”×éÓĆ¼×Č©·Ø½ųŠŠĮĖČēĻĀŹµŃé£ŗ
²½ÖčI ³ĘȔѳʷ1£®500 g”£
²½ÖčII ½«ŃłĘ·Čܽāŗó£¬ĶźČ«×ŖŅʵ½250 mLČŻĮæĘæÖŠ£¬¶ØČŻ£¬³ä·ÖŅ”ŌČ”£
²½ÖčIII ŅĘČ”25£®00 mLѳʷČÜŅŗÓŚ250 mL׶ŠĪĘæÖŠ£¬¼ÓČė10 mL 20£„µÄÖŠŠŌ¼×Č©ČÜŅŗ£¬Ņ”ŌČ”¢¾²ÖĆ5 minŗ󣬼ÓČė1~2µĪ·ÓĢŖŹŌŅŗ£¬ÓĆNaOH±ź×¼ČÜŅŗµĪ¶ØÖĮÖÕµć”£°“ÉĻŹö²Ł×÷·½·ØŌŁÖŲø“2“Ī”£
£Ø1£©øł¾Ż²½ÖčIIIĢīæÕ£ŗ
¢Ł¼īŹ½µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“µÓŗó£¬Ö±½Ó¼ÓČėNaOH±ź×¼ČÜŅŗ½ųŠŠµĪ¶Ø£¬Ōņ²āµĆѳʷ֊µŖµÄÖŹĮæ·ÖŹż________(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±)”£
¢Ś×¶ŠĪĘæÓĆÕōĮóĖ®Ļ“µÓŗó£¬Ė®Ī“µ¹¾”£¬ŌņµĪ¶ØŹ±ÓĆČ„NaOH±ź×¼ČÜŅŗµÄĢå»ż_______(Ģī”°Ę«“ó”±”¢”°Ę«Š””±»ņ”°ĪŽÓ°Ļģ”±)
¢ŪµĪ¶ØŹ±±ßµĪ±ßŅ”¶Æ׶ŠĪĘ棬ŃŪ¾¦Ó¦¹Ū²ģ____________”£
A£®µĪ¶Ø¹ÜÄŚŅŗĆęµÄ±ä»Æ B£®×¶ŠĪĘæÄŚČÜŅŗŃÕÉ«µÄ±ä»Æ
¢ÜµĪ¶Ø“ļµ½ÖÕµćŹ±ĻÖĻó£ŗ__________________________________________________”£
£Ø2£©µĪ¶Ø½į¹ūČēĻĀ±ķĖłŹ¾£ŗ
| µĪ¶Ø “ĪŹż | “ż²āČÜŅŗµÄĢå»ż /mL | ±ź×¼ČÜŅŗµÄĢå»ż/mL | |
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ||
| 1 | 25£®00 | 1£®02 | 21£®03 |
| 2 | 25£®00 | 2£®00 | 21£®99 |
| 3 | 25£®00 | 0£®20 | 20£®20 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĢśŹĒČĖĢå²»æÉȱɣµÄĪ¢ĮæŌŖĖŲ£¬ÉćČėŗ¬ĢśµÄ»ÆŗĻĪļæɲ¹³äĢś”£”°ĖŁĮ¦·Ę”±ŹĒŹŠ³”ÉĻŅ»ÖÖ³£¼ūµÄ²¹ĢśŅ©Ę·£¬ĻĀ±ķŹĒĖµĆ÷ŹéµÄ²æ·ÖÄŚČŻ”£
[¹ęøń]Ćæʬŗ¬ēśēźĖįŃĒĢś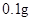 [ŹŹÓ¦Ö¢]ÓĆӌȱĢśŠŌʶŃŖÖ¢£¬Ō¤·Ą¼°ÖĪĮĘÓĆ”£ [ÓĆĮæÓĆ·Ø]³ÉČĖŌ¤·ĄĮæ 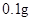 /ČÕ£¬³ÉČĖÖĪĮĘĮæ /ČÕ£¬³ÉČĖÖĪĮĘĮæ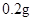 ”Ŗ ”Ŗ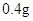 /ČÕ”£ /ČÕ”£Š”¶łÓĆĮæŌ¤·ĄĮæ 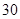 ”Ŗ ”Ŗ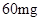 /ČÕ£¬ÖĪĮĘĮæ /ČÕ£¬ÖĪĮĘĮæ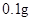 ”Ŗ ”Ŗ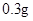 /ČÕ /ČÕ[Öü²Ų]±Ü¹ā”¢ĆÜ·ā”¢ŌŚøÉŌļ“¦±£“ę”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
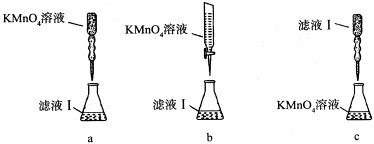
°×²ĖÖŠŗ¬ÓŠøĘĢśµČŌŖĖŲ£¬Ä³»ÆѧŠ”×éÉč¼ĘČēĻĀ·½°ø²ā¶ØøɰײĖÖŠøĘŌŖĖŲµÄÖŹĮæ·ÖŹż”£Ź×ĻČČ”10.00gøɰײĖŅ¶£¬×ĘÉÕµĆ°×²Ė»Ņ·Ū½ųŠŠĻĀĮŠŹµŃé£ŗ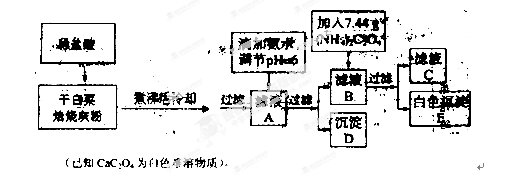
£Ø1£©ŹµŃéĒ°ŅŖĻČ½«øɰײĖŅ¶ŃłĘ·øßĪĀ×ĘÉճɻŅ·Ū£¬ĘäÖ÷ŅŖÄæµÄŹĒŹ¹ŃłĘ·ÖŠµÄÓŠ»śĪļĶźČ«·Ö½ā£¬Ź¹øɰײĖŅ¶ÖŠµÄøĘ”¢ĢśŌŖĖŲČܽāĶźČ«£¬×ĘÉÕÓƵ½µÄ²æ·ÖŅĒĘ÷ÓŠ
A£®ŪįŪö B£®Õō·¢Ćó C£®²£Į§°ō D£®ÄąČż½Ē
£Ø2£©Š“³ö“ÓĀĖŅŗA”ś³ĮµķDµÄĄė×Ó·“Ó¦·½³ĢŹ½ ”£
£Ø3£©ÓĆKMnO4±ź×¼ČÜŅŗµĪ¶ØĀĖŅŗC£ŗĻČ½«ĀĖŅŗCĻ”ŹĶÖĮ500 mL£¬ŌŁČ”ĘäÖŠµÄ25£®00 mLČÜŅŗ£¬ÓĆĮņĖįĖį»Æŗó£¬ÓĆ0.100 0 mol”¤L-1”ƵÄKMnO4±ź×¼ČÜŅŗµĪ¶Ø£¬ÖÕµćŹ±ĻūŗÄKMnO4ČÜŅŗ10.00mL”£
·¢ÉśµÄ·“Ó¦ĪŖ£ŗ
¢ŁµĪ¶ØµÄ¹ż³ĢÖŠ£¬Ķ¬Ń§ĆĒ·¢ĻÖŅ»øöĻÖĻó£ŗĻņCČÜŅŗÖŠ¼ÓČėµŚŅ»µĪKMnO4ČÜŅŗŹ±£¬ŠčŅŖÕńŅ”׶ŠĪĘæ½Ļ³¤Ź±¼ä²ÅÄÜĶŹÉ«£¬µ±ČÜŅŗĶŹÉ«ŗó£¬ŌŁµĪČėKMnO4ČÜŅŗ£¬ŌņŃøĖŁĶŹÉ«£¬Ö±ÖĮ“ļµ½ÖÕµć£»ĪŖĮĖ¼ÓæģµĪČėµŚŅ»µĪKMnO4ČÜŅŗŹ±µÄĶŹÉ«ĖŁ¶Č£¬æɲÉČ”µÄ·½·ØŹĒ .£ØŃ”ŌńŗĻŹŹµÄŃ”Ļī£©
A£®ŹŹµ±¼ÓČČ׶ŠĪĘæÄŚČÜŅŗ B£®ŌŚ×¶ŠĪĘæÄŚ¼ÓÉŁĮæĖ®
C£®ŌŚ×¶ŠĪĘæÄŚ¼ÓŹŹĮæŅŅ“¼ D£®ŌŚ×¶ŠĪĘæÄŚ¼ÓČė¼øµĪMnSO4ČÜŅŗ
¢ŚÅŠ¶ĻµĪ¶Ø“ļµ½ÖÕµćµÄ·½·ØŹĒ ”£
£Ø4£©ŌøɰײĖŅ¶ÖŠøĘŌŖĖŲµÄÖŹĮæ·ÖŹżĪŖ ”£
£Ø5£©ĪŖ±£Ö¤ŹµŃé¾«Č·¶Č£¬³ĮµķD¼°EŠčŅŖ·Ö±šĻ“µÓ£¬²¢½«Ļ“µÓŅŗ×ŖŅĘ»ŲÄøŅŗÖŠ£¬ŹŌÅŠ¶Ļ³ĮµķDŅŃ¾Ļ“µÓøɾ»µÄ·½·ØŹĒ ”£Čē¹ū³ĮµķEĪ“Ļ“µÓ£¬»ņĪ“½«Ļ“µÓŅŗ×ŖŅĘ»ŲÄøŅŗ£¬Ōņ²āµĆµÄøĘŌŖĖŲÖŹĮæ·ÖŹż £ØĢī”°Ę«øß”±”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com