
��ҵ��ұ����ͭ��mCu2O��nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���塣
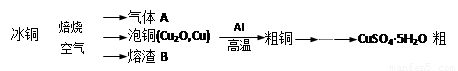
���������գ�
��1������A�еĴ�����Ⱦ���ѡ�������Լ��е� ������ţ����ա�
a. ŨH2SO4 b. ŨHNO3 c. NaOH��Һ d. ��ˮ
��2����ϡH2SO4 ��������B��ȡ����������Һ���μ� �����������ƣ���Һ��ʺ�ɫ��˵����Һ�д���Fe3+��������Һ�л�����Fe2+�ķ����� ��ע���Լ�������
|

��3����ͭұ����ͭ�Ļ�ѧ����ʽ�� ��
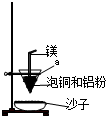
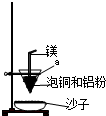
��4��װ����þ���������� ����ͭ�����ۻ������渲��������ɫ����a��
a�� (������)��ɳ���ܷ�ˮ�� (��ܡ����ܡ�)��
��5���õζ����ⶨCuSO4��5H2O�ĺ�����ȡa g�������100 mL��Һ��ȡ20.00mL��c mol /L �ζ���(H2Y2�C���ζ����������ʷ�Ӧ)�ζ����յ㣬���ĵζ���bmL���ζ���Ӧ��Cu2+ + H2Y2�C��CuY2�C+ 2H+����CuSO4��5H2O���������ı���ʽ�� ��
��6�����в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
a���ζ��ٽ��յ�ʱ����ϴƿ�е�����ˮϴ�µζ��ܼ���ڵİ�α�Һ����ƿ��
b���ζ���������ˮϴ�Ӻ�ֱ��ע�����Һ��ȡ20.00mL���еζ�
c���ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ
��1��c d ��2�֣�
��2�����軯�� ��1�֣�
��ȡ������Һ���μӼ��θ�����ص�������Һ����ɫ��ȥ��˵����Һ�д���Fe2+��2�֣�
��3��3Cu2O+2Al 6Cu+ Al2O3 ��1�֣�
6Cu+ Al2O3 ��1�֣�
��4��ȼ�շų��������ȣ�������»���������ȼ������1�֣� ����أ�1�֣� ���ܣ�1�֣�
��5��5bc/4a ��2�֣�
��6��c ��1�֣�
��������
�����������1�����ݱ�ͭ��mCu2O��nFeS�������Ԫ�ؿ�֪����ͭ��mCu2O��nFeS���ڿ�����ȼ�����ɵ�����AӦ����SO2���塣SO2�Ǵ�����Ⱦ����������������˿���������SO2���Լ�������������Һ��ˮ��Ũ���������SO2��Ũ��������SO2�����ɴ�����Ⱦ��NO2��������ȷ�Ĵ�ѡcd��
��2�����������ӵ��Լ���KSCN��Һ�������������Ӽ��黹ԭ�ԣ����Լ�����Һ�л������������ӵIJ�������ȡ������Һ���μӼ��θ�����ص�������Һ����ɫ��ȥ��˵����Һ�д���Fe2+��
��3������װ��ͼ��֪���÷�Ӧ�����ȷ�Ӧ����ѧ����ʽ��3Cu2O+2Al 6Cu+ Al2O3��
6Cu+ Al2O3��
��4�����ȷ�Ӧ��Ҫ���£���þȼ�տ��Էų�����������������þ����������ȼ�շų��������ȣ�������»���������ȼ���������������ȷ�Ӧ�л���Ҫ��ȼ������أ�������ͭ�����ۻ������渲��������ɫ����a������ء��������ȷ�Ӧ��ų�������������ʹ���ɵĽ����ۻ��������ˮ����ɳ�ӣ��ݻ��������������Բ�����������ɳ�ӡ�
��5�����ĵζ��������ʵ�����0.001bcmol������ݵζ���ӦCu2+ + H2Y2�C��CuY2�C+ 2H+��֪��20.00ml����ͭ��Һ������ͭ�����ʵ�����0.001bcmol����100ml��Һ������ͭ�����ʵ�����0.005bcmol������CuSO4��5H2O���������ı���ʽ��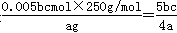 ��
��
��6�� a���ζ��ٽ��յ�ʱ����ϴƿ�е�����ˮϴ�µζ��ܼ���ڵİ�α�Һ����ƿ�У�������ȷ�IJ����������Ӱ�죻b���ζ���������ˮϴ�Ӻ�ֱ��ע�����Һ��ȡ20.00mL���еζ�����Һ�൱�ڱ�ϡ�ͣ�������ͭ�����ʵ������䣬��˽�����䣻c���ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ����˵�����ĵζ�����������ӣ��������ͭ�����ʵ������ӣ��ⶨ���ƫ�ߣ���ѡc��
���㣺����SO2β���������������Լ��������ӵļ��飻���ȷ�Ӧ���ζ���Ӧ�����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ҵ��ұ����ͭ��mCu2O?nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮
��ҵ��ұ����ͭ��mCu2O?nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮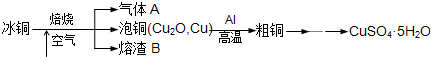
| ||
| ||
| ��� |
| 5bc |
| 4a |
| 5bc |
| 4a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ҵ��ұ�������ý�̿��ԭ������ | B��CO2��N02��SO2���ᵼ��������γ� | C�����ά������ϩ����֬���Ǹ߷��ӻ����� | D����ͨ����ˮ���պ����ð�Ĥ�����ķ�������PM2.5��������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ���������2013��߿�һģ��ѧ���� ���ͣ�058
��ҵ��ұ����ͭ(mCu2O��nFeS)�ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮

���������գ�
1������A�еĴ�����Ⱦ���ѡ�������Լ��е�________(�����)���գ�
a��ŨH2SO4
b��ŨHNO3
c��NaOH��Һ
d����ˮ
2����ϡH2SO4��������B��ȡ����������Һ���μ�________(����������)��Һ��ʺ�ɫ��˵����Һ�д���Fe3+��������Һ�л�����Fe2+�ķ�����________(ע���Լ�������)��ʵ���ҿ�������ͼ��װ�������ͭұ����ͭ�ķ�
Ӧ��

3����ͭұ����ͭ�Ļ�ѧ����ʽ��________��
4��װ����þ����������________����ͭ�����ۻ������渲��������ɫ
����a��a��________(������)��ɳ���ܷ�ˮ��________(��ܡ����ܡ�)��
5���õζ����ⶨCuSO4��5H2O�ĺ�����ȡa g�������100 mL��Һ��
ȡ20.00 mL��c mol/L�ζ���(H2Y2�����ζ����������ʷ�Ӧ)�ζ����յ㣬���ĵζ���b mL�ζ���Ӧ��Cu2+��H2Y2��![]() CuY2����2H+����CuSO4��5H2O���������ı���ʽ��________��
CuY2����2H+����CuSO4��5H2O���������ı���ʽ��________��
6�����в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���________��
a���ζ��ٽ��յ�ʱ����ϴƿ�е�����ˮϴ�µζ��ܼ���ڵİ�α�Һ����ƿ��
b���ζ���������ˮϴ�Ӻ�ֱ��ע�����Һ��ȡ20.00 mL���еζ�
c���ζ�ǰ���ζ��ܼ�������ݣ��ζ���������ʧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ҵ��ұ����ͭ��mCu2O?nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮
��ҵ��ұ����ͭ��mCu2O?nFeS���ɵõ���ͭ�����Դ�ͭΪԭ���Ʊ�����ͭ���壮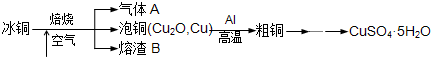
 ?CuY2-+2H+����CuSO4?5H2O���������ı���ʽ��______��
?CuY2-+2H+����CuSO4?5H2O���������ı���ʽ��______���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com