
 =0.0142mol��
=0.0142mol��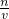 ���ɵø���Һ���ʵ���Ũ��=
���ɵø���Һ���ʵ���Ũ��= =14.2mol?L-1��
=14.2mol?L-1��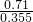 =2mol?L-1
=2mol?L-1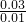 =3mol?L-1��
=3mol?L-1�� =1mol?L-1��
=1mol?L-1��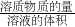 ����c=
����c=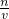 ��
��

 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010���㽭ʡ������ѧ�߶���ѧ�����п��Ի�ѧ�� ���ͣ������
��������������Ϣ�ƶ����ʵ���ɻ�ṹ��
����֪��ѧʽΪC5H9Cl��ij���ӵ�����̼ԭ�Ӻ���ԭ��һ������ͬһƽ���ϣ�д���÷��ӵĽṹ��ʽ�� ��
��������ͼ��ʾά����A�ķ��ӽṹ��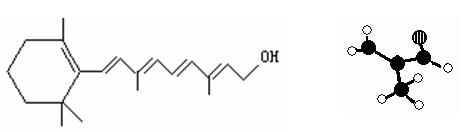
ά����A�ķ���ʽ ��άA���� ��
A������ B���������л��� C����״������ D�������廯���� E������
�DZ�������������������ij��Ʒֻ��C��H��O����Ԫ�أ������ģ��������ͼ��ʾ��ͼ��������֮������ߴ�����ѧ�����絥����˫���ȣ��ò�Ʒ�ļ���ʽΪ ����˴Ź��������� �������塣
��ij�л����������̼���⡢������Ԫ����ɣ�����Է�������С��150������֪����������������Ϊ50�����������̼ԭ�ӵĸ������Ϊ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�ij��ж��������������ϣ����л�ѧ�Ծ���������9�·ݣ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com