

»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ź¢ÅØH2SO4µÄ×°ÖĆÓĆĶ¾ŹĒ £¬Ź¢NaOHČÜŅŗµÄ×°ÖĆÓĆĶ¾ŹĒ ”£
£Ø2£©ŅĒĘ÷BÖŠŠč¼ÓČėŹŌ¼ĮµÄĆū³Ę£Ø»ņ»ÆѧŹ½£©ŹĒ£ŗ £¬Ėł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ£ŗ ”£
£Ø3£©ŅĒĘ÷CÖŠŠč¼ÓČėŹŌ¼ĮµÄĆū³Ę£Ø»ņ»ÆѧŹ½£©ŹĒ£ŗ £¬ĘäÄæµÄŹĒ ”£
£Ø4£©°“ĘųĮ÷·½ĻņĮ¬½Óø÷ŅĒĘ÷£¬ÓĆ×ÖÄø±ķŹ¾½ÓæŚµÄĮ¬½ÓĖ³Šņ£ŗg”Ŗab”Ŗ ”£
£Ø5£©ÄÜÖ¤Ć÷»ģŗĻĘųÖŠŗ¬ÓŠCOµÄŹµŃéŅĄ¾ŻŹĒ ”£
£Ø6£©ÄÜÖ¤Ć÷»ģŗĻĘųÖŠŗ¬ÓŠH2µÄŹµŃéŅĄ¾ŻŹĒ ”£
(1)³żČ„Ė®ÕōĘų ³żČ„CO2
(2)Ńõ»ÆĶ(CuO)
CuO+H2=Cu+H2O CuO+CO=Cu+CO2
(3)ĪŽĖ®ĮņĖįĶ(CuSO4) ¼ģŃéH2O
(4)(g”Ŗab)”Ŗkj”Ŗhi”Ŗcd(»ņdc)”Ŗfe”Ŗlm
(5)Ō»ģŗĻĘųÖŠµÄCO2Ņѱ»³żČ„£¬ĘäÖŠCOÓėCuO·“Ӧɜ³ÉµÄCO2Ź¹³ĪĒåŹÆ»ŅĖ®±ä»ė×Ē
(6)Ō»ģŗĻĘųÖŠµÄH2OŅѱ»³żČ„£¬ĘäÖŠH2ÓėCuO·“Ӧɜ³ÉµÄH2OŹ¹ĪŽĖ®ĮņĖįĶÓÉ°×É«±äĪŖĄ¶É«
½āĪö£ŗ±¾ĢāĪŖŅ»×ŪŗĻæ¼²éĪļÖŹµÄÖʱø”¢³żŌÓ”¢¼ģŃ飬ŅĒĘ÷µÄĮ¬½ÓµČÄŚČŻµÄ×ŪŗĻŹµŃéĢā”£¼ÓČČĖ®²śÉśµÄĖ®ÕōĘųĶعżAÖŠ½¹Ģæ²śÉśCO”¢H2”¢CO2”¢H2O(g)µČĘųĢ壬Ź×ĻČ¾¹żNaOHČÜŅŗ³żČ„CO2(±ÜĆāøÉČÅCOµÄ¼ģŃé)£¬ŌŁĶعżÅØH2SO4³żČ„Ė®ÕōĘų£¬ÅųżĮĖ¶ŌH2¼ģŃéµÄøÉČÅ£¬ŌŁĶعżŹ¢ÓŠ×ćĮæCuOµÄB¹Ü·¢Éś·“Ó¦H2+CuO![]() Cu+H2O£¬CO+CuO
Cu+H2O£¬CO+CuO![]() Cu+CO2£¬ŌŁ½«²śÉśµÄĘųĢåĶعżŹ¢ÓŠCuSO4µÄøÉŌļ¹ÜC£¬æÉÓÉ°×É«·ŪÄ©±äĄ¶Č·¶Øŗ¬H2£¬×īŗó½«ĘųĢåĶØČė³ĪĒåŹÆ»ŅĖ®£¬±ä»ė×Ē£¬ŌņÖ¤Ć÷ŗ¬CO£»ÓÉĻ“Ęų×°ÖĆ³¤½ų¶Ģ³ö£¬øÉŌļ¹Ü×°ÖĆ“ó½ųŠ”³ö²»ÄŃČ·¶ØĘä½ÓæŚĖ³Šņ”£
Cu+CO2£¬ŌŁ½«²śÉśµÄĘųĢåĶعżŹ¢ÓŠCuSO4µÄøÉŌļ¹ÜC£¬æÉÓÉ°×É«·ŪÄ©±äĄ¶Č·¶Øŗ¬H2£¬×īŗó½«ĘųĢåĶØČė³ĪĒåŹÆ»ŅĖ®£¬±ä»ė×Ē£¬ŌņÖ¤Ć÷ŗ¬CO£»ÓÉĻ“Ęų×°ÖĆ³¤½ų¶Ģ³ö£¬øÉŌļ¹Ü×°ÖĆ“ó½ųŠ”³ö²»ÄŃČ·¶ØĘä½ÓæŚĖ³Šņ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| øßĪĀ |
| Ņ»¶ØĢõ¼ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
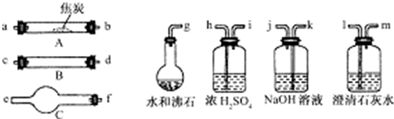
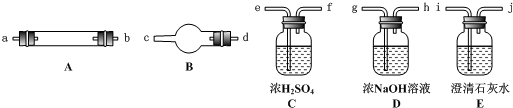
Ė®ÕōĘųĶعż×ĘČČµÄ½¹Ģæŗó£¬Į÷³öĘųĢåµÄÖ÷ŅŖ³É·ÖŹĒCOŗĶH2£¬»¹ÓŠCO2ŗĶĖ®ÕōĘųµČ£®ĒėÓĆĻĀĶ¼ÖŠĢį¹©µÄŅĒĘ÷£¬Ń”Ōń±ŲŅŖµÄŹŌ¼Į£¬Éč¼ĘŅ»øöŹµŃ飬֤Ć÷ÉĻŹö»ģŗĻĘųĢåÖŠÓŠCOŗĶH2£®£Ø¼ÓČČ×°ÖĆŗĶµ¼¹ÜµČŌŚĶ¼ÖŠĀŌČ„£©

»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Ź¢ÅØH2SO4µÄ×°ÖĆÓĆĶ¾ŹĒ________£¬Ź¢NaOHČÜŅŗµÄ×°ÖĆÓĆĶ¾ŹĒ________£®
£Ø2£©ŅĒĘ÷BÖŠŠč¼ÓČėŹŌ¼ĮµÄĆū³Ę£Ø»ņ»ÆѧŹ½£©ŹĒ________£¬Ėł·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ____________________£®
£Ø3£©ŅĒĘ÷CÖŠŠč¼ÓČėŹŌ¼ĮµÄĆū³Ę£Ø»ņ»ÆѧŹ½£©ŹĒ________£¬ĘäÄæµÄŹĒ__________________£®
£Ø4£©°“ĘųĮ÷·½ĻņĮ¬½Óø÷ŅĒĘ÷£¬ÓĆ×ÖÄø±ķŹ¾½ÓæŚµÄĮ¬½ÓĖ³Šņ£ŗg”Ŗab”Ŗ___________________£®
£Ø5£©ÄÜÖ¤Ć÷»ģŗĻĘųĢåÖŠŗ¬ÓŠCOµÄŹµŃéŅĄ¾ŻŹĒ__________________________________£®
£Ø6£©ÄÜÖ¤Ć÷»ģŗĻĘųĢåÖŠŗ¬ÓŠH2µÄŹµŃéŅĄ¾ŻŹĒ____________________________£®
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com