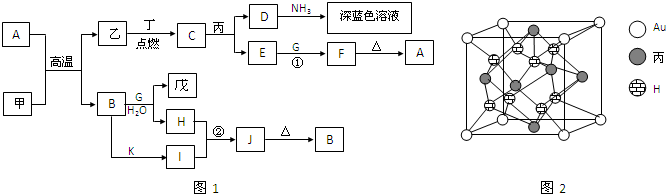
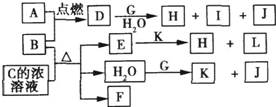
�⣺��J��Ԫ��ԭ�Ӻ���ֻ��һ������֪JΪH
2��I����ɫ��ӦΪ��ɫ������I��NaԪ�أ��ɽ���B��A������ȼ�ղ����ػ�ɫ�̿�֪AΪCl
2��BΪFe��Cu����DΪFeCl
3��CuCl
2����
��D+G+H
2O��H+I+J�����I��NaԪ��֪GΪ�����ƣ���H
2O+G��K+J��H
2��������KΪNaOH��D��ˮ��Һ���ػ�ɫ����DΪFeCl
3����ˮ��Һ��Na�ķ�ӦΪ��6Na+2FeCl
3+6H
2O�T2Fe��OH��
3��+6NaCl+3H
2����FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��ӦΪSO
2����CΪH
2SO
4��EΪFe
2��SO
4��
3��HΪFe��OH��
3��LΪNaCl��
��1�������Ϸ�����֪��KΪNaOH������Ϊ�������ƣ�������ѧ��Ϊ���Ӽ����ۼ����ʴ�Ϊ���������ƣ����Ӽ������ۼ���
��2������D��ˮ��Һ���ػ�ɫ��DΪFeCl
3�����Ȼ�����Һ�м�������ƣ����Ⱥ�ˮ��Ӧ�����������ƺ��������������ƺ��Ȼ�����Ӧ��������������������FeCl
3��ˮ��Һ��Na��Ӧ�����ӷ���ʽΪ��Fe
3++6H
2O+6Na=2Fe��OH��
3��+6Na
++3H
2����2Na+2H
2O�T2Na
++2OH
-+H
2����Fe
3++3OH
-=Fe��OH��
3����
�ʴ�Ϊ��2Fe
3++6H
2O+6Na=2Fe��OH��
3��+6Na
++3H
2����2Na+2H
2O�T2Na
++2OH
-+H
2����Fe
3++3OH
-=Fe��OH��
3����
������BΪFe��CΪH
2SO
4������������Ũ�����ڼ��������·�Ӧ�����������Ͷ��������ˮ����Ӧ�Ļ�ѧ����ʽΪ��2Fe+6H
2SO
4��Ũ��

Fe
2��SO
4��
3+3SO
2��+6H
2O��
�ʴ�Ϊ��2Fe+6H
2SO
4��Ũ��

Fe
2��SO
4��
3+3SO
2��+6H
2O��
��3��������FΪSO
2ͨ��һ����KΪNaOH��ˮ��Һ�У���Ӧ��������������Һ����������������Һ�����������������Һ�и�����Ũ��һ����������й�ϵʽΪ��
A����Һ��һ�����ڵ���غ㣺c��Na
+��+c��H
+��=c��OH
-��+c��HSO
3-��+2c��SO
32-������Һ��һ�����ڵ���غ㣬��Aһ����
B.2c��Na
+��=3c��H
2SO
3��+3c��HSO
3-��+3c��SO
32-�������ɵ�����Һ��Na
2SO
3ʱ�����غ�Ϊc��Na
+��=2c��H
2SO
3��+2c��HSO
3-��+2c��SO
32-��������NaHSO
3��Һ���������غ�Ϊ����c��Na
+��=c��H
2SO
3��+c��HSO
3-��+c��SO
32-��������Na
2SO
3��NaHSO
3�Ļ����Һ�����ڵ������غ�Ϊ��2c��Na
+��=3c��H
2SO
3��+3c��HSO
3-��+3c��SO
32-������B��һ����
C��c��Na
+����c��HSO
3-����c��SO
32-����c��H
2SO
3��������Ũ�ȴ�С������������Һ�е�����Ũ�ȴ�С�������ɵ�������������Һʱ������Ũ�ȴ�СΪ��c��Na
+����c��SO
32-����c��HSO
3-����c��H
2SO
3������C��һ����
D��c��OH
-����c��H
+����Һ�����ɵ�����Һ��Na
2SO
3��ǿ��������ˮ���Լ��ԣ�c��OH
-����c��H
+��������NaHSO
3����Һ������������ӵ���̶ȴ���������������ӵ�ˮ�⣬��Һ�����ԣ�c��OH
-����c��H
+������D��һ����
�ʴ�Ϊ��A��
��������״����2.24L��SO
2ͨ��150mL1mol?L
-1��NaOH��Һ�У�n��SO2��=0.1 mol��n��NaOH��=0.15mol��n��SO
2����n��NaOH��=2��3����Ӧ���������������ƺ����������ƵĻ����Һ����Ӧ�Ļ�ѧ����ʽΪ��2SO
2+3NaOH=Na
2SO
3+NaHSO
3+H
2O����������Һ�и�����Ũ�ȹ�ϵ�����������ϵʽ��
A��һ��������Һ�еĵ���غ㣬��A���ϣ�
B��Na
2SO
3��NaHSO
3�Ļ����Һ�����ڵ������غ�Ϊ��2c��Na
+��=3c��H
2SO
3��+3c��HSO
3-��+3c��SO
32-������B���ϣ�
C����Ӧ�õ�����ͬŨ�ȵ��������ƺ����������ƵĻ����Һ����Һ��������������ӵ缫�̶ȴ��������������ˮ��̶ȣ�����Һ�����ԣ���Һ������Ũ�ȴ�СΪc��Na
+����c��SO
32-����c��HSO
3-����c��H
2SO
3������C�����ϣ�
D����Ӧ�õ�����ͬŨ�ȵ��������ƺ����������ƵĻ����Һ����Һ��������������ӵ缫�̶ȴ��������������ˮ��̶ȣ�����Һ�����ԣ�c��OH
-����c��H
+������D�����ϣ�
�ʴ�Ϊ��AB��
��������J��Ԫ��ԭ�Ӻ���ֻ��һ������֪JΪH
2��I����ɫ��ӦΪ��ɫ������I��NaԪ�أ��ɽ���B��A������ȼ�ղ����ػ�ɫ�̿�֪AΪCl
2��BΪFe��Cu����DΪFeCl
3��CuCl
2����
��D+G+H
2O��H+I+J�����I��NaԪ��֪GΪ�����ƣ���H
2O+G��K+J��H
2��������KΪNaOH��D��ˮ��Һ���ػ�ɫ����DΪFeCl
3����ˮ��Һ��Na�ķ�ӦΪ��
6Na+2FeCl
3+6H
2O�T2Fe��OH��
3��+6NaCl+3H
2����FΪ��ɫ���д̼�����ζ���壬����ʹƷ����Һ��ɫ��ӦΪSO
2����CΪH
2SO
4��EΪFe
2��SO
4��
3��HΪFe��OH��
3��LΪNaCl���Դ˽����⣮
���������⿼��������ƶϣ���Һ������Ũ�ȴ�С�Ƚϡ�����غ㡢�����غ��Ӧ�ã���Ŀ�Ѷ��еȣ�������Ĺؼ�����ȷ�ƶ����ʵ����࣬�������ʵ����ʽ��

 �ش��������⣺
�ش��������⣺ Fe2��SO4��3+3SO2��+6H2O��
Fe2��SO4��3+3SO2��+6H2O�� Fe2��SO4��3+3SO2��+6H2O��
Fe2��SO4��3+3SO2��+6H2O��

 �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�